| [1] Nilsson ES,Urist MR,Dawson EG,et al.Bone repair induced by bone morphogenetic protein in ulnar defects in dogs.J Bone Joint Surg. 1986;68B: 635-642. [2] Burchardt H.Related Articles,The biology of bone graft repair.Clin Orthop. 1983;(174):28-42. [3] Enneking WF,Eady JL,Burchardt H.Autogenous cortical bone grafts in the reconstruction of segmental skeletal defects.J Bone Joint Surg Am. 1980;62(7): 1039-1058. [4] Pazzaglia UE.Periosteal and endosteal reaction to reaming and nailing: the possible role of revascularization on the endosteal anchorage of cementless stems. Biomaterials. 2012;17(10):1009-1014. [5] Weng D,Hürzeler MB,Quiñones CRet al.Contribution of the periosteum to bone formation in guided bone regeneration. A study in monkeys.Clin Oral Implants Res.2014;11(6):546-554. [6] Aitasalo K,Lehtinen R.The influence of a free periosteal transplant on bone healing in the irradiated tibia. A radiological histological and biochemical study on rabbits. Scand J Plast Reconstr Surg. 1985;19(3): 237-244. [7] Zhang C,Hu YY,Cui FZ,et al.A study on a tissue- engineered bone using rhBMP - 2 induced periosteal cells with a porous nano -hydroxyapatite/collagen/poly (L-lactic acid) scaffold.Biomed Mater.2006;1(2):56-62. [8] Schmidt BL,Kung L,Jones C,et al.Induced osteogenesis by periosteal distraction. J Oral Maxillofac Surg.2002;60(10):1170-1175. [9] Sencimen M,Aydintug YS,Ortakoglu K,et al. Histomorphometrical analysis of new bone obtained by distraction osteogenesis and osteogenesis by periosteal distraction in rabbits.Int J Oral Maxillofac Surg.2007;36(3):235-242. [10] Glowacki J,Shusterman EM,Troulis M,et al.Distraction osteogenesis of the porcine mandible: histomorphometric evaluation of bone.Plast Reconstr Surg.2004;113(2):566-573. [11] 曾融生,任材年,于秦曦.珊瑚人工骨作为颌面骨修复材料的初步报告[J].中华口腔医学杂志, 1991,26(6):345-347. [12] Souyris F, Pellequer C.Coral,a new biomedical material: experimental and first clinical investigation on Madreporaria.J Maxillfac Surg.1985;13:64-69. [13] 郑有华,任材年,张志光,等.珊瑚人工骨/自体骨髓复合移植修复颌骨缺损临床应用[J].中国现代医学杂志, 2005, 15(8):1227-1230 [14] 郑有华,任材年,陈小华,复合珊瑚骨修复颌骨实验性骨缺损[J].临床口腔医学杂志,1996,12(3):143-145. [15] Chen F,Mao T,Tao K,et al.Bone graft in the shape of human mandibular condyle reconstruction via seeding marrow-derived osteoblasts into porous coral in a nude mice model.J Oral Maxillofac Surg. 2002;60:1155-1159. [16] Guillemin G,Patat JL.The use of coral as a bone graft substitutes.J Biomed Mater Res.1987;21:557-567. [17] Weibrich G,Kleis WK,Hafner G.Growth factor levels in the platelet-rich plasma produced by 2 different methods:curasan-type PRP Kit versus PCCS PRP system.Int J Oral Maxillofac Implants. 2002;17(2): 184-190. [18] Cenni E,Granchi D,Vancini M,et al.Platelet release of transforming growth factor-β and β-thromboglobulin after in vitro contract with acrylic bone cements. Biomaterials.2002;23(6):1479-1484. [19] Casap N,Venezia NB,Wilensky A,et al.VEGF facilitates periosteal distraction- induced osteogenesis in rabbits: a micro-computerized tomography study. Tissue Eng Part A.2008;14(2):247-253. [20] Gruber R,Mayer C,Bobacz K,et al.Effects of cartilage-derived morphogenetic proteins and osteogenic protein-1 on osteochondrogenic differentiation of periosteum-derived cells. Endocrinology. 2014;142(5):2087-2094. [21] Nakahara H,Goldberg VM,Caplan AI. Cultured- expanded periosteal-derived cells exibit osteochondrogenic potential in porous calcium phosphate ceramics in vivo.Clin Orthop Relat Res. 2013;(276):291-298. [22] Breitbart AS,Grande DA,Kessler R,et al.Tissue engineered bone repair of calvarial defects using cultured periosteal cells.Plast Reconstr Surgy.2014 Mar;101(3):567-74. [23] Sakata Y,Ueno T,Kagawa T,et al.Osteogenic potential of cultured human periosteum-derived cells - a pilot study of human cell transplantation into a rat calvarial defect model.J Craniomaxillofac Surg. 2014;34(8): 461-465. [24] Zhu SJ,Choi BH,Huh JY,et al.A Comparative qualitative histological analysis of tissue-engineered bone using bone marrow messenchmal stem cells,alveolar bone cells and periosteal cells.Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2013; 101(2):164-169. [25] Mizuno H,Hata K,Kojima K,et al.A novel approach to regenerating periodontal tissue by grafting autologous cultured periosteum.Tissue Eng. 2015;12(5):1227-1335. [26] Schilephake H.Bone growth factors in maxillofacial skeletal reconstruction.Int J Oral Maxillofac Surg. 2002; 31(5):469-484. [27] 陈鹏,刘冰.活性纳米羟基磷灰石复合胶原/聚乳酸材料修复颅骨极限缺损的实验研究[J].中国修复重建外科杂志, 2007,21(11):1191-1195. [28] Karageorgiou V,Kaplan D.Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2015;26(27): 5474-5479. [29] Gauthier O,Bouler JM,Aguado E,et al.Macroporous biphasic calcium phosphate ceramics: influence of macropore diameter and macroporosity percentage on bone ingrowth. Biomaterials.1998;19(1-3):133-139. [30] 马红梅,吴琳,艾红军,等. 纳米羟基磷灰石/胶原/聚乳酸支架材料对人成骨细胞早期附着影响的实验研究[J].生物医学工程与临床,2007,11(3):161-163. [31] Weibrich G,Hansen T,Kleis W, et al.Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration.Bone.2014;34(4):665-671. [32] Kim SG,Chung CH,Kim YK,et al.Use of particulate dentin-plaster of Paris combination with /without platelet-rich plasma in the treatment of bone defects around implants.Int J Oral Maxillofac Implants. 2012; 17(1):86-94. [33] Anitua E,Sanchez M,Nurden AT,et al.New insights into and novel applications for platelet-rich fibrin therapies.Trends Biotechnol.2006;24(5):227-234. [34] Jensen TB,Rahbek O,Overgaard S,et al.Platelet-rich plasma and fresh frozen bone allograft as enhancement of implant fixation: An experimental study in dogs. J Orthop Res. 2004;22(3):653-658. [35] Jensen TB,Rahbek O,Overgaard S,et al.Platelet-rich plasma and fresh frozen bone allograft as enhancement of implant fixation: An experimental study in dogs.J Orthop Res.2004;22(3):653-658. [36] Kitoh H,Kitakoji T,Tsuchiya H,et al.Transplantation of marrow-derived mesenchymal stem cells and platelet-rich plasma during distraction osteogenesis--a preliminary result of three cases. Bone. 2004;35(4):892-898. [37] Robiony M,Polini F,Costa F,et al.Osteogenesis distraction and platelet-rich plasma for bonerestoration of the severely atrophic mandible: preliminary results.J Oral Maxillofac Surg.2002;60(6):630-635. [38] Zechner W,Tangl S,Tepper G,et al.Influence of platelet-rich plasma on osseous healing of dental implants: a histologic and hisgomorphometric study in minipigs.Int J Oral Maxillofac Implants. 2003;18(1): 15-22. [39] Choi BH,Zhu SJ,Kim BY,et al.Effect of platelet-rich plasma (PRP) concentration on the viability and proliferation of alveolar bone cells: an in vitro study.Int J Oral Maxillofac Surg.2005;34(4):420-424. [40] Kim SG,Chung CH,Kim YK,et al.Use of particulate dentin-plaster of Paris combination with /without platelet-rich plasma in the treatment of bone defects around implants.Int J Oral Maxillofac Implants. 2002; 17(1):86-94. Anitua E, Sanchez M,Nurden AT,et al.New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol.2006;24(5):227-23 |
.jpg)
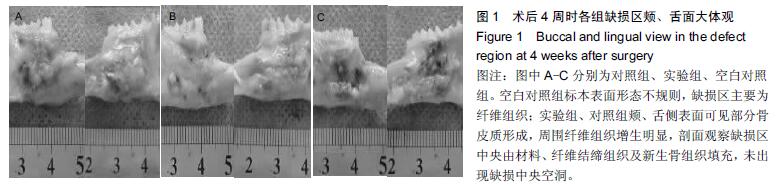
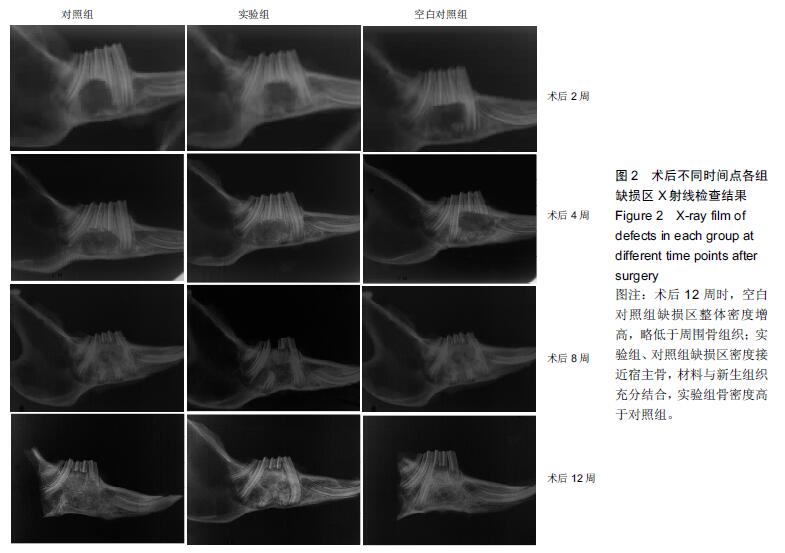
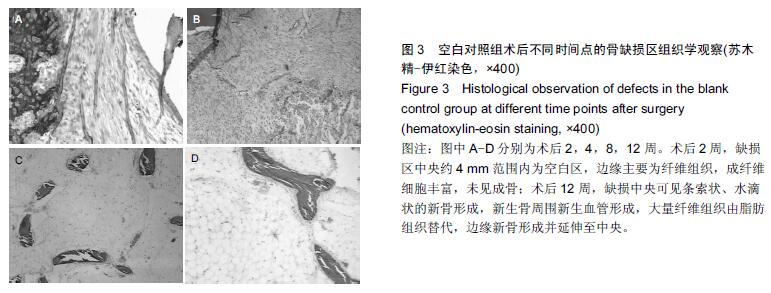
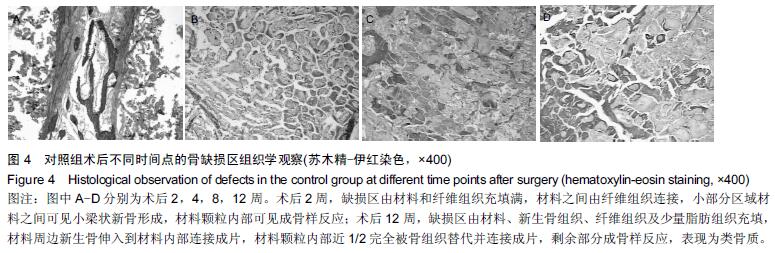
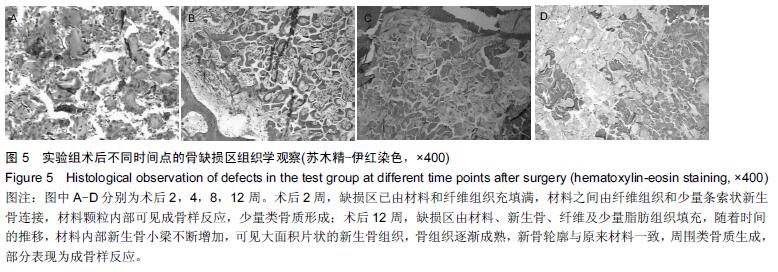
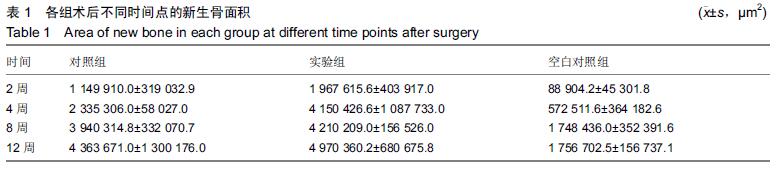
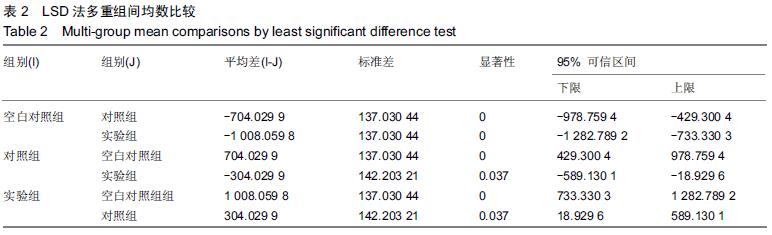
.jpg)