| [1] Staiger MP, Pietak AM, Huadmai J, et al. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials. 2006;27(9):1728-1734. [2] 谢兴文,黄晋,李宁,等.镁及镁合金植入体在骨科临床中的应用与进展[J].中国组织工程研究, 2012,16(39): 7317-7321. [3] Han P, Tan M, Zhang S, et al. Shape and site dependent in vivo degradation of Mg-Zn pins in rabbit femoral condyle. Int J Mol Sci. 2014;15(2):2959-2970. [4] Ratna Sunil B, Sampath Kumar TS, Chakkingal U, et al. In vitro and in vivo studies of biodegradable fine grained AZ31 magnesium alloy produced by equal channel angular pressing. Mater Sci Eng C Mater Biol Appl. 2016;59:356-367. [5] Lindtner RA, Castellani C, Tangl S, et al. Comparative biomechanical and radiological characterization of osseointegration of a biodegradable magnesium alloy pin and a copolymeric control for osteosynthesis. J Mech Behav Biomed Mater. 2013;28:232-243. [6] 王树峰,李春荣,王程越,等.微弧氧化AZ31镁合金的生物相容性[J].中国组织工程研究, 2012,16(38): 7101-7106. [7] Kraus T, Fischerauer SF, Hänzi AC, et al. Magnesium alloys for temporary implants in osteosynthesis: in vivo studies of their degradation and interaction with bone. ActaBiomater. 2012;8(3):1230-1238. [8] 薛静,彭江,汪爱媛,等.活体小动物Micro-CT动态评价大鼠股骨牵张成骨[J].中国矫形外科杂志,2010,(9): 752-758. [9] 彭江,汪爱媛,孙明学,等.Micro-CT在松质骨结构研究中的应用[J].中国矫形外科杂志,2005,(11):859-861. [10] Fischerauer SF, Kraus T, Wu X, et al. In vivo degradation performance of micro-arc-oxidized magnesium implants: a micro-CT study in rats. Acta Biomater. 2013;9(2):5411-5420. [11] Schambach SJ, Bag S, Schilling L, et al. Application of micro-CT in small animal imaging. Methods. 2010; 50(1):2-13. [12] Clark DP, Badea CT. Micro-CT of rodents: State-of-the-art and future perspectives. Phys Med. 2014;(30):619-634. [13] 张涛,武肖娜,尹庆水,等.镁合金AZ31B材料表性与成骨细胞的黏附[J].中国组织工程研究,2013,17(12): 2123-2130. [14] 齐峥嵘,张强,殷毅,等.可降解镁合金作为骨植入材料的体内研究进展[J].中国修复重建外科杂志,2012,26(11): 1381-1386. [15] Grünewald TA, Ogier A, Akbarzadeh J, et al. Reaction of bone nanostructure to a biodegrading Magnesium WZ21 implant: a scanning small-angle X-ray scattering time study. Acta Biomater. 2015. [16] Brooks EK, Der S, Ehrensberger MT. Corrosion and mechanical performance of AZ91 exposed to simulated inflammatory conditions. Mater Sci Eng C Mater Biol Appl. 2016;60:427-436. [17] Witte F. The history of biodegradable magnesium implants: a review. Acta Biomater. 2011;6:1680-1692. [18] Walker J, Shadanbaz S, Woodfield TB, et al. Magnesium biomaterials for orthopedic application: a review from a biological perspective. J Biomed Mater Res B Appl Biomater. 2014;102(6):1316-1331. [19] Vladimirov BV, Krit BL, Lyudin VB, et al. Microarc oxidation of magnesium alloys: a review. Surface Eng App Electrochem. 2014;50(3):195-232. [20] 张佳,宗阳,付彭怀,等.镁合金在生物医用材料领域的应用及发展前景[J].中国组织工程研究与临床康复,2009, 13(29):5747-575. [21] Zhen Z, Liu XL, Huang T, et al. BHemolysis and cytotoxicity mechanisms of biodegradable magnesium and its alloys. Mater Sci Eng C Mater Biol Appl. 2015; 46:202-206. [22] Pompa L, Rahman ZU, Munoz E, et al. Surface characterization and cytotoxicity response of biodegradable magnesium alloys. Mater Sci Eng C Mater Biol Appl. 2015;49:761-768. [23] Wang DW, Cao Y, Qiu, H, et al. Improved blood compatibility of Mg-1.0Zn-1.0Ca alloy by micro-arc oxidation. J Biomed Mater Res A. 2011;99(2):166-172. [24] Zhang EL, Xu LP, Yu GN, et al. In vivo evaluation of biodegradable magnesium alloy bone implant in the first 6 months implantation. J Biomed Mater Res A. 2009;90(3):882-893. [25] Schillinga T, Brandesa G, Tudorache I, et al. In vivo degradation of magnesium alloy LA63 scaffolds for temporary stabilization of biological myocardial grafts in a swine model. Biomed Tech (Berl). 2013;58(5): 407-416. [26] Seitz JM, Eifler R, Bach FW, et al. Magnesium degradation products: effects on tissue and human metabolism. J Biomed Mater Res A. 2014;102(10): 3744-3753. [27] Chen YJ, Xu ZG, Smith C, et al. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014;10(11): 4561-4573. [28] Waizy H, Diekmann J, Weizbauer A, et al. In vivo study of a biodegradable orthopedic screw (MgYREZr-alloy) in a rabbit model for up to12months. J Biomater Appl. 2014;28(5):667-675. [29] Clark DP, Badea CT. Micro-CT of rodents: State-of-the-art and future perspectives. Physica Medica.2014;(30):619-634. [30] Vanderoost J, Harry van GL. From histology to micro-CT: measuring and modeling resorption cavities and their relation to bone competence. World J Radiol. 2014;6(9):643-656. [31] Zhao D, Wang T, Kuhlmann J, et al. In vivo monitoring the biodegradation of magnesium alloys with an electrochemical H2 sensor. Acta Biomater. 2016. [32] Schaller B, Saulacic N, Imwinkelried T, et al. In vivo degradation of magnesium plate/screw osteosynthesis implant systems: Soft and hard tissue response in a calvarial model in miniature pigs. J Craniomaxillofac Surg. 2016;44(3):309-317. [33] Meininger S, Mandal S, Kumar A, et al. Strength reliability and in vitro degradation of three-dimensional powder printed strontium-substituted magnesium phosphate scaffolds. Acta Biomater. 2016;31:401-411. [34] Durisin M, Reifenrath J, Weber CM, et al. Biodegradable nasal stents (MgF2 -coated Mg-2 wt % Nd alloy)-A long-term in vivo study. J Biomed Mater Res B Appl Biomater. 2015. [35] Ikeo N, Nakamura R, Naka K, et al. Fabrication of a magnesium alloy with excellent ductility for biodegradable clips. Acta Biomater. 2016;29:468-476. |
.jpg)
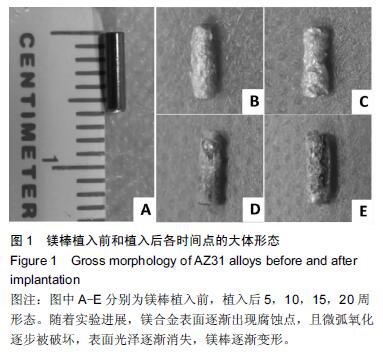
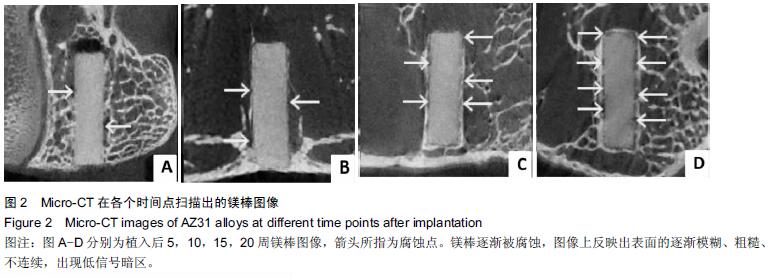
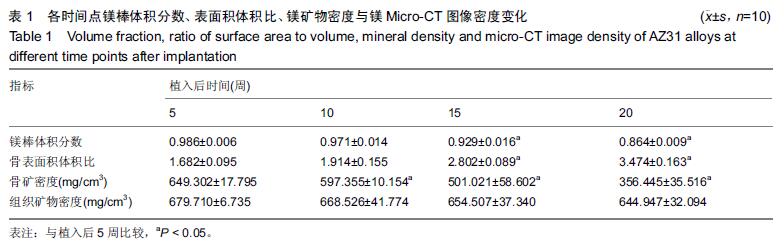
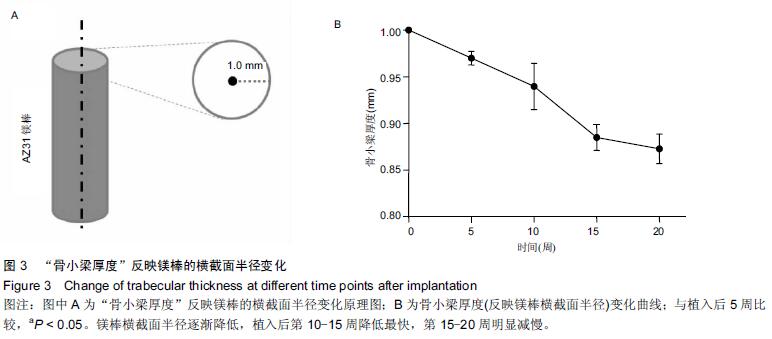
.jpg)