| [1] Greco E, Nanji S, Bromberg IL, et al.Predictors of peri-opertative morbidity and liver dysfunction after hepatic resection in patients with chronic liver disease. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2011;13(8):559-565.
[2] El-Karaksy HM, El-Shabrawi MM, Mohsen NA, et al. Study of predictive value of pediatric risk of mortality (PRISM) score in children with end stage liver disease and fulminant hepatic failure. Indian journal of pediatrics. 2011;78(3):301-306.
[3] 马海芬,保志军.肝脏衰老与肝脏疾病[M].肝脏,2013,18(1): 55-57.
[4] Starzl TE, Marchioro TL, Vonkaulla KN, et al. Homotransplantation of the Liver in Humans. Surgery, gynecology & obstetrics. 1963;117:659-676.
[5] 唐晖. 肝移植术后缺血性胆道损伤研究进展[J]. 器官移植, 2014, 5(2):123-127.
[6] Hansen T, Hollemann D, Pitton MB, et al. Histological examination and evaluation of donor bile ducts received during orthotopic liver transplantation--a morphological clue to ischemic-type biliary lesion? Virchows Arch. 2012;461(1):41-44.
[7] 金鑫,史宪杰,王茂强,等.肝移植术后缺血型胆道病变的病因及防治[J].中华医学杂志,2011,91(4):251-255.
[8] Farid WR, de Jonge J, Zondervan PE, et al.Relationship Between the Histological Appearance of the Portal Vein and Development of Ischemic-Type Biliary Lesions after Liver Transplantation. Liver Transplantation. 2013; 19(10):1088-1098.
[9] Tsukada N, Ackerley CA, Phillips MJ. The Structure and Organization of the Bile Canalicular Cytoskeleton with Special Reference to Actin and Actin-Binding Proteins. Hepatology. 1995;21(4):1106-1113.
[10] 黎一鸣,刘继东,吉鸿,等.大鼠肝脏缺血再灌注对胆小管纤维形肌动蛋白微丝的影响[J].西安交通大学学报:医学版, 2009, 30(2): 210-213,239.
[11] Benkoel L, Dodero F, Hardwigsen J, et al.Effect of ischemia- reperfusion on bile canalicular F-actin microfilaments in hepatocytes of human liver allograft - Image analysis by confocal laser scanning microscopy. Digestive Diseases and Sciences. 2001;46(8):1663-1667.
[12] Nagai H, Kato A, Kimura F, et al.Endothelin-1 aggravates hepatic ischemia/reperfusion injury during obstructive cholestasis in bile duct ligated mice. J Surg Res. 2010; 162(1):46-53.
[13] Nieuwenhuijs VB, De Bruijn MT, Padbury RT, et al. Hepatic ischemia-reperfusion injury: roles of Ca2+ and other intracellular mediators of impaired bile flow and hepatocyte damage.Dig Dis Sci. 2006;51(6):1087-1102.
[14] Sudo R, Kohara H, Mitaka T, et al.Coordinated movement of bile canalicular networks reconstructed by rat small hepatocytes. Ann Biomed Eng. 2005;33(5):696-708.
[15] van der Heijden M, Versteilen AM, Sipkema P, et al.Rho- kinase-dependent F-actin rearrangement is involved in the inhibition of PI3-kinase/Akt during ischemia-reperfusion-induced endothelial cell apoptosis. Apoptosis. 2008;13(3):404-412.
[16] Hutchins BI, Klenke U, Wray S.Calcium Release-Dependent Actin Flow in the Leading Process Mediates Axophilic Migration. J Neurosci. 2013; 33(28):11361-11371.
[17] Ritchey L, Chakrabarti R. Aurora A kinase modulates actin cytoskeleton through phosphorylation of Cofilin: Implication in the mitotic process. Bba-Mol Cell Res. 2014;1843(11): 2719-2729.
[18] Huang J, Xie LD, Luo L, et al. Silencing heat shock protein 27 (HSP27) inhibits the proliferation and migration of vascular smooth muscle cells in vitro. Molecular and cellular biochemistry. 2014, 390(1-2):115-121.
[19] Kostenko S, Johannessen M, Moens U. PKA-induced F-actin rearrangement requires phosphorylation of Hsp27 by the MAPKAP kinase MK5. Cell Signal. 2009;21(5):712-718.
[20] Anne OS, Bernheim-Groswasser A. Reconstitution of Actin-based Motility by Vasodilator-stimulated Phosphoprotein (VASP) Depends on the Recruitment of F-actin Seeds from the Solution Produced by Cofilin. Journal of Biological Chemistry. 2014;289(45):31274-31286.
[21] Chen CK, Benchaar SA, Phan M, et al. Cofilin-induced changes in F-actin detected via cross-linking with benzophenone-4- maleimide. Biochemistry. 2013;52(32):5503-5509.
[22] 杨怡,张德添,张飒,等.培养细胞透射电镜超薄切片制备方法[J]. 电子显微学报,2004, 23(4):506-506.
[23] Liu Y, Wang J, Yang P, et al. Delayed rearterialization unlikely leads to nonanastomotic stricture but causes temporary injury on bile duct after liver transplantation.Transplant international : official journal of the European Society for Organ Transplantation. 2015;28(3):341-351.
[24] Sun Y, Meng GM, Guo ZL, et al. Regulation of heat shock protein 27 phosphorylation during microcystin-LR-induced cytoskeletal reorganization in a human liver cell line. Toxicology letters. 2011; 207(3):270-277.
[25] Hambarde S, Singh V, Chandna S. Evidence for involvement of cytosolic thioredoxin peroxidase in the excessive resistance of Sf9 Lepidopteran insect cells against radiation-induced apoptosis. PloS one.2013; 8(3):e58261.
[26] Evertsson K, Fjallstrom AK, Norrby M, et al.p38 mitogen-activated protein kinase and mitogen-activated protein kinase-activated protein kinase 2 (MK2) signaling in atrophic and hypertrophic denervated mouse skeletal muscle. J Mol Signal. 2014;9(1):2.
[27] Collins P, Jones C, Choudhury S, et al.Increased expression of uncoupling protein 2 in HepG2 cells attenuates oxidative damage and apoptosis. Liver International. 2005;25(4): 880-887.
[28] Huh YH, Kim SH, Chung KH, et al. Swiprosin-1 modulates actin dynamics by regulating the F-actin accessibility to cofilin. Cell Mol Life Sci. 2013;70(24):4841-4854.
[29] Lian JP, Marks PG, Wang JY, et al. A protein kinase from neutrophils that specifically recognizes Ser-3 in cofilin. J Biol Chem. 2000;275(4):2869-2876.
[30] 雷艳萍,何洁,苏琦. Cofilin与分化的研究进展[J].中国药理学通报, 2014,30(2):164-167.
[31] Hsieh YC, Rao YK, Wu CC, et al. Methyl Anteinate A from Antrodia camphorata Induces Apoptosis in Human Liver Cancer Cells through Oxidant-Mediated Cofillin- and Bax-Triggered Mitochondrial Pathway. Chem Res Toxicol. 2010;23(7):1256-1267. |
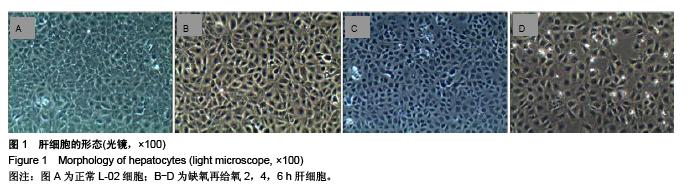
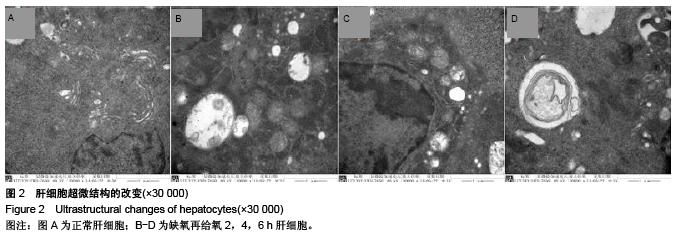
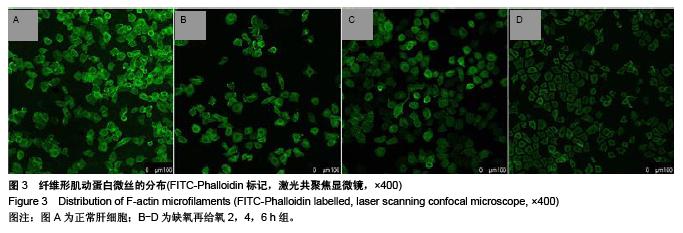
.jpg)
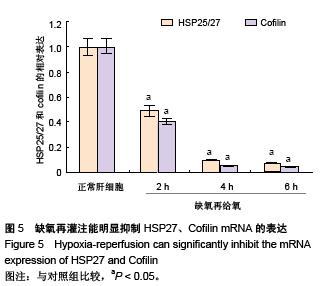
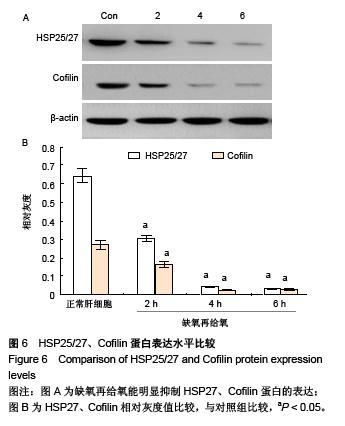
.jpg)