设计:对比观察动物实验。
时间及地点:于2008年4月至2011年8月在华北煤炭医学院动物实验室完成。
材料:
实验动物及分组:新西兰大耳白兔30只,雌雄不拘,体质量2.5-2.7 kg,由山西省医用组织库实验动物中心提供。随机分成10组,每组3只,用来研究植入后1,2,3,6,9,12,15,18,25,30 d,加替沙星-聚癸二酸酐局部缓释系统的体内释药特性;各组动物在实验前均常规喂养2周。
加替沙星-聚癸二酸酐局部缓释系统体内释药特性实验的试剂与仪器:
实验方法:
加替沙星-聚癸二酸酐局部缓释制剂的制作:将聚癸二酸酐与加替沙星原料药按加替沙星含量为20%比例在研钵内充分混合,研磨,加入自制模具内,压紧,然后按空白聚癸二酸酐棒的制备方法制备,制成质量(40.0±1.0) mg、大小为直径3 mm×长5 mm的药棒,加替沙星含量为20%。将加替沙星-聚癸二酸酐缓释制剂密封,60Co辐照灭菌,辐照剂量为20 kGy,-40 ℃保存备用。
加替沙星-聚癸二酸酐局部缓释制剂的体内释药实验:实验前1 d,将30只新西兰大耳白兔右侧髂腰部及右后肢用脱毛液脱毛,随机分成10组,每组3只,麻醉后取仰卧位,在右侧膝关节上3 cm处做股骨前外侧纵行切口,切开皮肤、皮下骨膜,用3 mm钻头的手钻,在该处钻2个纵向相连、部分重叠的骨洞,用尖嘴咬骨钳将骨洞修整成3 mm×6 mm大小的骨窗。将加替沙星-聚癸二酸酐缓释制剂放入髓腔内,用骨蜡封闭骨窗,逐层缝合伤口,术后回笼饲养。
血标本采集:术后1,2,3,6,9,12,15,18,25,30 d处死动物,每组动物处死前无菌心脏采血3 mL,肝素抗凝,4 000 r/min离心15 min,吸取上清液,-20 ℃存放待测。
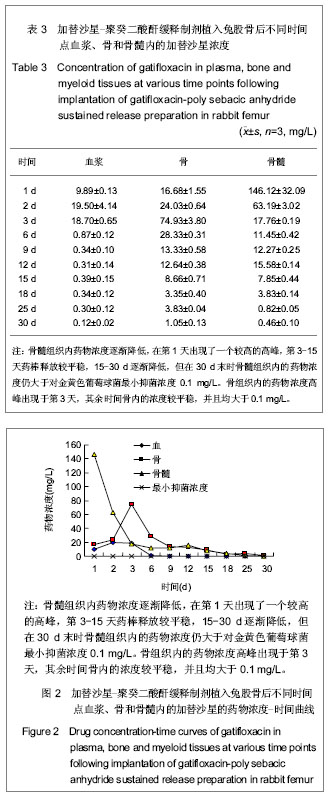
骨组织及骨髓组织标本的采集:各组动物采集完心脏血后,空气栓塞法处死各组动物,取出右侧股骨,用尖嘴咬骨钳咬开股骨,以离药棒两端1 cm处为中心点,在中心点两侧0.5 cm宽度内,采取松质骨及骨干皮质骨标本,骨髓标本取自距药棒边缘0.5 cm处的干骺端。用研钵、研棒进一步粉碎骨标本,称骨粉100 mg,加入含有2 mL 0.1 mol/L的PBS(pH值=7.0)试管中,充分匀浆10 min,4 000 r/min离心15 min,吸取上清液,-20 ℃存放待测。取骨髓组织100 mg,加入含有2 mL 0.1 mol/L的PBS(pH值=7.0)试管中,充分匀浆10 min,4 000 r/min离心15 min,吸取上清液,-20 ℃存放待测。
高效液相色谱法测定各标本内加替沙星浓度[1-3]:
溶液的配置:①pH值7.0 PBS:在100 mL容量瓶内,加磷酸二氢钾0.68 g,0.1 mol/L氢氧化钠溶液 29.10 mL,用蒸馏水稀释至100 mL。②加替沙星标准品储备液:精密称取加替沙星标准品4.20 mg,加入到100 mL容量瓶内,然后加入乙腈-水(3∶10)至刻度,配制成42 mg/L加替沙星标准品储备液。③左氧氟沙星标准品储备液:精密称取左氧氟沙星标准品25.00 mg,加入到25 mL容量瓶内,然后加入乙腈-水(3∶10)至刻度,配制成1 g/L加替沙星标准品储备液。
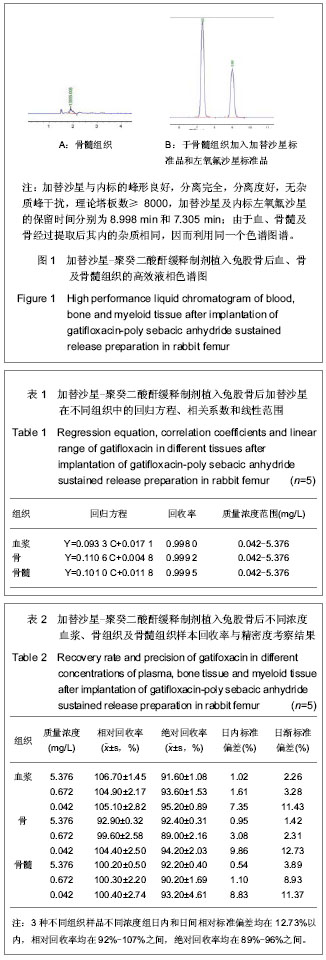
色谱条件:高效液相色谱仪;色谱柱(Venusil MP-C18 4.6 mm×250 mm,5 µm,安捷伦科技有限公司);流动相为乙腈-水-十二烷基硫酸钠(500∶500∶3.5),用磷酸调pH值为3.0;流速1.0 mL/min;检测波长290 nm;柱温35 ℃;进样量20 µL。
方法的专属性:在上述色谱项条件下测得色谱图。
样品预处理:精密量取空白血浆、骨与骨髓组织匀浆液各0.25 mL,分别加入内标溶10.0 µL,pH值7.0 PBS 0.25 mL和二氯甲烷5.0 mL混合后,漩涡振荡 5 min,4 000 r/min离心15 min,吸取下层有机相,然后再加入二氯甲烷5.0 mL,混合提取1次,吸取下层有机相,放入另一试管中,在40 ℃水浴内用氮气将二氯甲烷溶液吹干,残渣加1 mL乙腈-水(3∶10)溶解,取20 µL进样。
标准曲线的配制:于空白血浆、空白骨与骨髓匀浆液中加入加替沙星标准品,使质量浓度分别为5.376,2.688,1.344,0.672,0.336,0.168,0.084,0.042 mg/L的标准血浆样品,按上述“样品预处理”方法操作进行,分别以对照品峰面积与内标峰面积比值(Y)对加替沙星浓度(C)进行回归计算,求其直线回归方程。
回收率实验:配制血浆、骨与骨髓组织匀浆液样品5.376,0.672,0.042 mg/L 3种不同质量浓度各5份,按照“样品预处理”方法进行,以实测浓度与理论浓度之比计算相对回收率。取加替沙星标准品溶液适量于试管内,用乙腈-水(3∶10)配成5.376,0.672,0.042 mg/L工作液,每种质量浓度做5份,取20 µL进样分析,记录加替沙星峰面积,计算绝对回收率。
精密度实验:分别配制血浆、骨与骨髓组织匀浆液样品5.376,0.672,0.042 mg/L 3种不同质量浓度,进行日内和日间精密度考察。①日内精密度:日内配置高中低3种不同质量浓度样品,每种浓度做5份,每份样品测定2次,求其日内差。②日间精密度:每天配置高中低3种不同质量浓度样品,一次进行测定,连续配制测定5 d,求其日间差。根据公式计算日间差。
电镜观察加替沙星-聚癸二酸酐缓释制剂的表面及内部结构变化:将降解剩余的药棒常规方法制样,经过冷冻干燥、喷金,用JSM-6360LV扫描电镜观察加替沙星-聚癸二酸酐缓释制剂的表面和内部变化。
主要观察指标:①测定各组织内加替沙星浓度。②加替沙星-聚癸二酸酐缓释制剂植入前后的结构变化。

.jpg)
.jpg)