中国组织工程研究 ›› 2020, Vol. 24 ›› Issue (8): 1213-1217.doi: 10.3969/j.issn.2095-4344.1872
• 肌肉肌腱韧带组织构建 tissue construction of the muscle, tendon and ligament • 上一篇 下一篇
机械法与机械-酶消化法制备大鼠膈肌组织单细胞悬液的比较
张远圆1,张杉杉1,常 旭2,赵朋伟1,余孝先1,吴学东1
- 1大理大学第一附属医院暨临床医学研究中心小儿外科,云南省大理市 671000;2大理大学基础医学院,云南省大理市 671000
Preparing single cell suspension of rat diaphragm tissues:
mechanical grinding versus mechanical-enzymatic digestion
Zhang Yuanyuan1, Zhang Shanshan1, Chang Xu2, Zhao Pengwei1, Yu Xiaoxian1, Wu Xuedong1
- 1Department of Pediatric Surgery, the First Affiliated Hospital & Clinical Medical Research Center of Dali University, Dali 671000, Yunnan Province, China; 2School of Basic Medicine, Dali University, Dali 671000, Yunnan Province, China
摘要:
文题释义:
流式细胞分析技术:简称流式细胞术,是20世纪70年代初发展起来的一种在功能水平上可以对单个细胞或其他生物离子进行快速定量的一项新型分析技术和分选技术。它能够在短时间内高速分析上万个细胞,可以同时测量如细胞或粒子的体积、内部结构以及膜电位变化等生物或化学性质,进行多信息分析,具有快速、高灵敏、准确、多参数等特点。
单细胞悬液:是进行细胞体外培养和流式细胞分析的基础,即将组织分散成单个游离细胞,使其悬浮在液体培养基中,当然如外周血及骨髓标本等本身即为天然的单细胞悬液,这些细胞经过简单处理,就可以应用于流式分析、细胞分选、细胞培养等实验。如果2个或多个细胞粘连在一起或细胞碎片过多都将影响流式分析结果,进而导致实验失败。
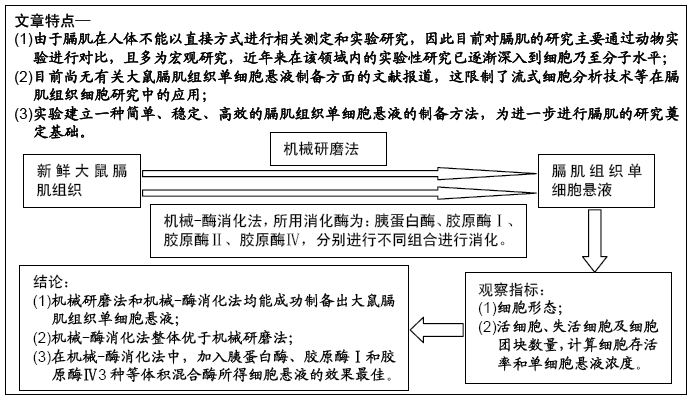
背景:流式细胞术作为目前先进的细胞分析技术,其研究基础是单细胞悬液,但目前尚无有关膈肌组织单细胞悬液制备方法的相关报道。
目的:探索应用机械法与机械-酶消化法分别制备大鼠膈肌单细胞悬液的可行性并比较2种方法获得单细胞的数量和活性。
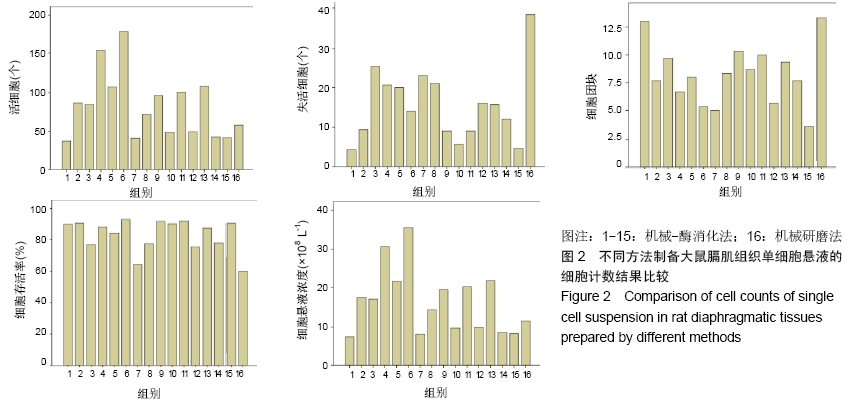
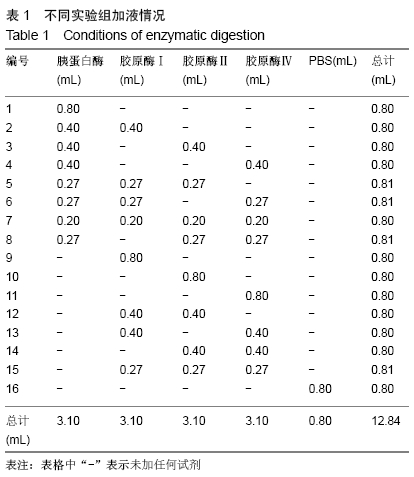
方法:以SD大鼠的新鲜膈肌组织为标本,在机械法的基础上,选用胰蛋白酶、胶原酶Ⅰ、胶原酶Ⅱ、胶原酶Ⅳ及其不同组合进行消化制备膈肌单细胞悬液,观察细胞形态并经锥虫蓝染色后测定细胞活性,对活细胞、失活细胞和细胞团块进行计数并计算出细胞存活率和单细胞悬液浓度,结果进行统计学比较分析。
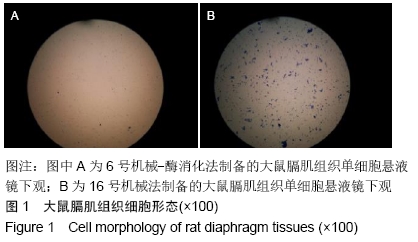
结果与结论:①机械-酶消化法所制得的单细胞悬液较机械研磨法细胞分散度好、形态完整、边界清楚、杂质及细胞碎片较少、背景较干净;②单纯机械研磨法制备的单细胞悬液活细胞数较低,失活细胞数较高,细胞存活率最低,团块较多;③在机械法的基础上,加入胰蛋白酶、胶原酶Ⅰ和胶原酶Ⅳ3种等体积混合酶所得单细胞悬液的活细胞数及悬液浓度最高,每0.1 g膈肌组织可获得(1.0-2.0)×106个细胞,细胞存活率较高,与单纯机械法获得单细胞悬液比较,在活细胞、失活细胞、细胞团块、细胞存活率及悬液浓度方面差异均有显著性意义(P < 0.05);其次是加入胰蛋白酶和胶原酶Ⅳ这2种等体积混合酶消化法,所得单细胞悬液浓度、细胞成活率及细胞团块方面也均较为理想;④结果表明,机械法和机械-酶消化法均能成功制备出大鼠膈肌单细胞悬液,机械-酶消化法优于单纯机械法,其中加入胰蛋白酶、胶原酶Ⅰ和胶原酶Ⅳ3种等体积混合酶的机械-酶消化法所得单细胞悬液效果最佳,是较优选的膈肌单细胞悬液制作方法。
ORCID: 0000-0002-4486-5917(张远圆)
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
中图分类号: