中国组织工程研究 ›› 2024, Vol. 28 ›› Issue (22): 3478-3483.doi: 10.12307/2024.534
• 组织工程骨材料 tissue-engineered bone • 上一篇 下一篇
大鼠硬脑膜对颅骨成骨增强的影响
安 冉1,2,3,4,邵 国5,张春阳2,3,4
- 1内蒙古科技大学包头医学院,内蒙古自治区包头市 014000;2内蒙古科技大学包头医学院第一附属医院神经外科,内蒙古自治区包头市 014010;3包头医学院神经外科疾病研究所(转化医学),内蒙古自治区包头市 014010;4内蒙古自治区骨组织再生与损伤修复工程技术中心,内蒙古自治区包头市 014010;5深圳市龙岗区第三人民医院转化医学中心,广东省深圳市 518100
Effect of dura mater on enhancement of cranial osteogenesis in rats
An Ran1, 2, 3, 4, Shao Guo5, Zhang Chunyang2, 3, 4
- 1Baotou Medical College of Inner Mongolia University of Science and Technology, Baotou 014000, Inner Mongolia Autonomous Region, China; 2Department of Neurosurgery, First Affiliated Hospital of Baotou Medical College of Inner Mongolia University of Science and Technology, Baotou 014010, Inner Mongolia Autonomous Region, China; 3Institute of Neurosurgical Diseases (Translational Medicine), Baotou Medical College, Baotou 014010, Inner Mongolia Autonomous Region, China; 4Inner Mongolia Autonomous Region Bone Tissue Regeneration and Damage Repair Engineering Technology Center, Baotou 014010, Inner Mongolia Autonomous Region, China; 5Translational Medicine Center of The Third People’s Hospital of Longgang District of Shenzhen City, Shenzhen 518100, Guangdong Province, China
摘要:
文题释义:
硬脑膜:脑表面有3层被膜,最外层是硬脑膜,向内依次是蛛网膜和软脑膜,由2层致密的胶原纤维构成,分别是靠近颅骨内表面的骨膜层和内层的脑膜层,它不仅参与保护脑组织,在颅骨的成骨分化和损伤修复过程中也发挥重要作用。骨矿化:指磷酸钙等无定形的无机矿物质以羟基磷灰石结晶形式沉积于骨有机质中,与有机质鳌合形成成熟骨体的生化过程。因此,骨矿化能力是成骨细胞成骨能力的主要标志,而矿化结节是成骨细胞分化成熟的标志,可采用茜素红等染色法观察。
背景:硬脑膜与颅骨在结构和功能上联系密切,原代提取硬脑膜和颅骨细胞并将二者共培养的研究几乎没有,利用原代细胞探究硬脑膜对颅骨的影响具有创新性,有望为临床治疗提供理论依据。
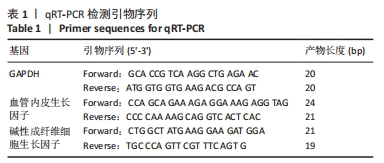
目的:原代提取大鼠硬脑膜和颅骨细胞,观察硬脑膜对颅骨增殖和分化能力的影响,初步了解Twist1在其中的作用。方法:利用酶解法与组织块法相结合的方法原代提取出生3 d内大鼠硬脑膜细胞和颅骨细胞,免疫荧光染色鉴定所提取细胞,茜素红染色鉴定与评估颅骨细胞及其矿化能力,real-time PCR检测硬脑膜细胞与颅骨细胞共培养后,颅骨细胞增殖与成骨相关基因表达及Twist1的表达。
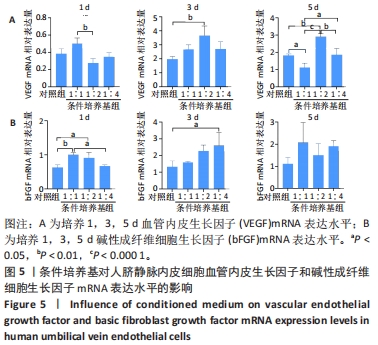
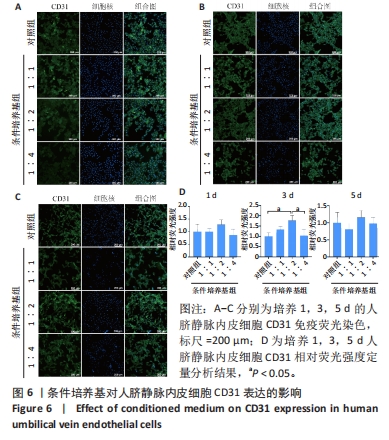
结果与结论:①形态学:所提取硬脑膜细胞形态特征与成纤维细胞一致,成骨细胞呈纺锤形。②细胞鉴定:免疫荧光染色显示,所提取硬脑膜细胞表达高水平的波形蛋白,颅骨细胞表达高水平的碱性磷酸酶;成骨诱导28 d颅骨细胞茜素红染色观察到明显的矿化结节。③real-time PCR检测显示,与对照组比较,共培养组PCNA、碱性磷酸酶、RUNX2 mRNA表达升高(P < 0.01);Twist1 mRNA表达降低(P < 0.01)。④结果表明,原代提取的颅骨细胞具有较强的矿化能力,硬脑膜是促进颅骨的生长发育与成骨分化的重要因素,且Twist1在该过程中发挥重要作用。
中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
中图分类号: