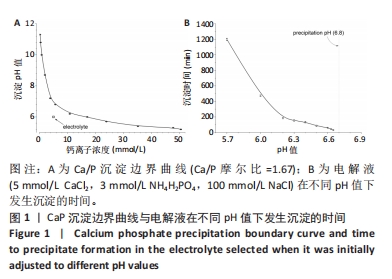
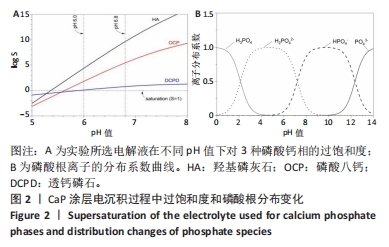
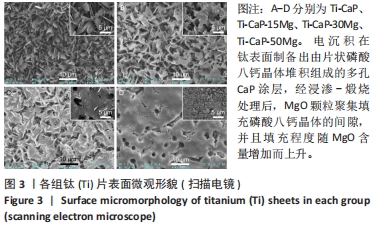
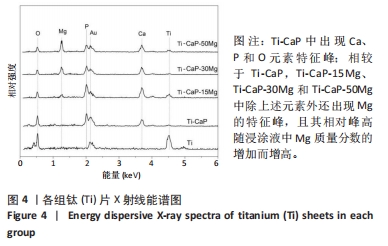
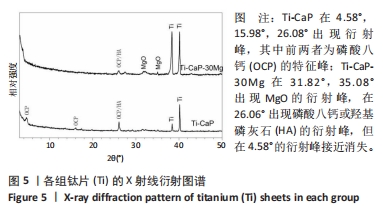
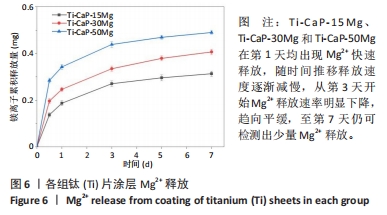
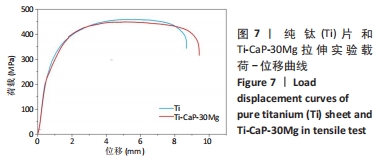
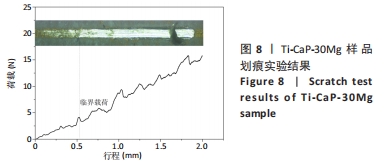
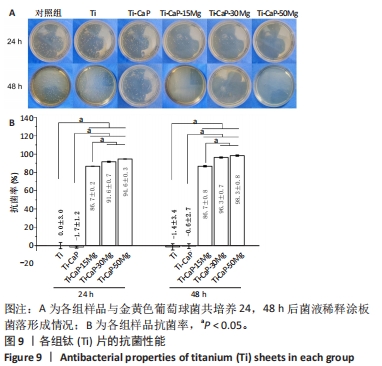
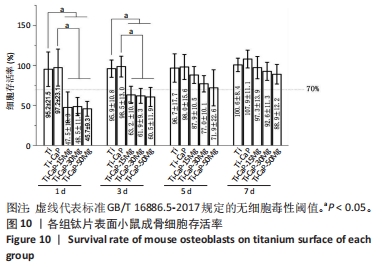
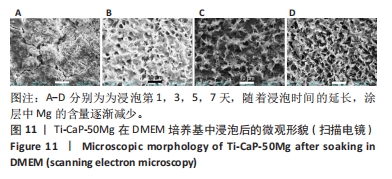
[1] KUMAR ST, DEVI SP, KRITHIKA C, et al. Review of metallic biomaterials in dental applications. J Pharm Bioallied Sci. 2020;12(Suppl 1):S14.
[2] ALVAND A, REZAPOOR M, PARVIZI J. The role of biomarkers for the diagnosis of implant-related infections in orthopaedics and trauma. Adv Exp Med Biol. 2017;971:69-79.
[3] NANA A, NELSON SB, MCLAREN A, et al. What’s new in musculoskeletal infection: update on biofilms. J Bone Joint Surg Am. 2016;98(14): 1226-1234.
[4] DONLAN RM. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002;8(9):881.
[5] NARAYANAN R, SESHADRI SK, KWON TY, et al.Calcium phosphate-based coatings on titanium and its alloys. J Biomed Mater Res B Appl Biomater. 2008;85(1):279-299.
[6] SUN L, BERNDT CC, GROSS KA, et al. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: a review. J Biomed Mater Res. 2001;58(5):570-592.
[7] HAMAGAMI J, ATO Y, KANAMURA K. Fabrication of highly ordered macroporous apatite coating onto titanium by electrophoretic deposition method. Solid State Ion. 2004;172(1-4):331-334.
[8] LOPEZ-HEREDIA MA, WEISS P, LAYROLLE P. An electrodeposition method of calcium phosphate coatings on titanium alloy. J Mater Sci Mater Med. 2007;18:381-390.
[9] KNUDSEN MB, THILLEMANN JK, JØRGENSEN PB, et al. Electrochemically applied hydroxyapatite on the cementless porous surface of Bi-Metric stems reduces early migration and has a lasting effect: an efficacy trial of a randomized five-year follow-up radiostereometric study. Bone Joint J. 2022;104(6):647-656.
[10] KARLOV AV, KHLUSOV IA, PONTAK VA, et al. Adhesion of Staphylococcus aureus to implants with different physicochemical characteristics. Bull Exp Biol Med. 2002;134:277-280.
[11] VOGELY HC, OOSTERBOS CJM, PUTS EWA, et al. Effects of hydroxyapatite coating on Ti‐6 Al‐4V implant‐site infection in a rabbit tibial model. J Orthop Res. 2000;18(3):485-493.
[12] LAZARINIS S, MÄKELÄ KT, ESKELINEN A, et al. Does hydroxyapatite coating of uncemented cups improve long-term survival? An analysis of 28,605 primary total hip arthroplasty procedures from the Nordic Arthroplasty Register Association (NARA). Osteoarthritis Cartilage. 2017;25(12):1980-1987.
[13] ALTOMARE L, VISAI L, BLOISE N, et al. Electrochemically deposited gentamicin-loaded calcium phosphate coatings for bone tissue integration. Int J Artif Organs. 2012;35(10):876-883.
[14] KOSE N, OTUZBIR A, PEKŞEN C, et al. A silver ion-doped calcium phosphate-based ceramic nanopowder-coated prosthesis increased infection resistance. Clin Orthop Relat Res. 2013;471:2532-2539.
[15] PIERRE C, BERTRAND G, PAVY I, et al. Antibacterial Electrodeposited Copper-Doped Calcium Phosphate Coatings for Dental Implants. J Funct Biomater. 2022;14(1):20.
[16] CHEN Z, MENG H, XING G, et al. Acute toxicological effects of copper nanoparticles in vivo. Toxicol Lett. 2006;163(2):109-120.
[17] VORMANN J. Magnesium: nutrition and metabolism. Mol Aspects Med. 2003;24(1-3):27-37.
[18] ARANCIBIA-HERNÁNDEZA YL, HERNÁNDEZ-CRUZA EY, PEDRAZA-CHAVERRIA J. Magnesium (Mg2+) Deficiency, Not Well-Recognized Non-Infectious Pandemic: Origin and Consequence of Chronic Inflammatory and Oxidative Stress-Associated Diseases. Cell Physiol Biochem. 2023;57(S1):1-23.
[19] 中国居民膳食常量元素参考摄入量[C]//中国营养学会微量元素营养分会.中国营养学会微量元素营养第十二次学术会议暨第六届微量元素营养分会会员大会论文集.四川西昌,2014:164.
[20] PATEL MK, ZAFARYAB M, RIZVI M, et al. Antibacterial and cytotoxic effect of magnesium oxide nanoparticles on bacterial and human cells. J Nanoeng Nanomfg. 2013;3(2):162-166.
[21] SAWAI J, KOJIMA H, IGARASHI H, et al. Antibacterial characteristics of magnesium oxide powder. World J Microbiol Biotechnol. 2000;16:187-194.
[22] BHATTACHARYA P, DEY A, NEOGI S. An insight into the mechanism of antibacterial activity by magnesium oxide nanoparticles. J Mater Chem B. 2021;9(26):5329-5339.
[23] COELHO CC, ARAÚJO R, QUADROS PA, et al. Antibacterial bone substitute of hydroxyapatite and magnesium oxide to prevent dental and orthopaedic infections. Mater Sci Eng C Mater Biol Appl. 2019;97: 529-538.
[24] 肖东琴,李文,段可,等.骨科植入金属材料表面电化学制备磷酸钙涂层方法及机理研究[J].西部医学,2023,35(8):1110-1116,1121.
[25] 武汉大学.分析化学[M].6版.北京:高等教育出版社,2016.
[26] RAMANUJAM K, SUNDRARAJAN M. Antibacterial effects of biosynthesized MgO nanoparticles using ethanolic fruit extract of Emblica officinalis. J Photochem Photobiol B. 2014;141:296-300.
[27] 黄珂,杨伏良,陈力学,等.划痕法测定TiAlN涂层结合强度的研究[J].表面技术,2013,42(5):107-111.
[28] 董自艳,戴翚,马仕洪,等.紫外-可见分光光度法快速确定细菌菌液的浓度[J].中国药品标准,2014(2):120-121.
[29] YADAV P, SAXENA KK. Effect of heat-treatment on microstructure and mechanical properties of Ti alloys: An overview. Mater Today Proc. 2020;26:2546-2557.
[30] DE ROOIJ JF, HEUGHEBAERT JC, NANCOLLAS GH. A pH study of calcium phosphate seeded precipitation. J Colloid Interface Sci. 1984;100(2): 350-358.
[31] BROWN WE. Crystallographic and chemical relations between octacalcium phosphate and hydroxyapatite. Nature. 1962;196:1050-1055.
[32] JEONG CH, KIM J, KIM HS, et al. Acceleration of bone formation by octacalcium phosphate composite in a rat tibia critical-sized defect. J Orthop Translat. 2022;37:100-112.
[33] JIN H, JIA D, YANG Z, et al. Fabrication and properties of embedded dense coating on direct ink writing Si2N2O porous ceramics. Surf Coat Technol. 2020;394:125801.
[34] SPIRANDELI BR, RIBAS RG, AMARAL SS, et al. Incorporation of 45S5 bioglass via sol-gel in β-TCP scaffolds: Bioactivity and antimicrobial activity evaluation. Mater Sci Eng C Mater Biol Appl. 2021;131:112453.
[35] SUZUKI O, SHIWAKU Y, HAMAI R. Octacalcium phosphate bone substitute materials: Comparison between properties of biomaterials and other calcium phosphate materials. Dent Mater J. 2020;39(2): 187-199.
[36] DONG C, HE G, ZHENG W, et al. Study on antibacterial mechanism of Mg (OH) 2 nanoparticles. Mater Lett. 2014;134:286-289.
[37] Liu Y, Liu Y, Li X, et al. Fabrication and research of Mg (OH) 2/PCL/PVP nanofiber membranes loaded by antibacterial and biosafe Mg (OH) 2 nanoparticles. Polym Test. 2022;112:107635.
[38] LORD MS, FOSS M, BESENBACHER F. Influence of nanoscale surface topography on protein adsorption and cellular response. Nano Today. 2010;5(1):66-78.
[39] HICKEY DJ, ERCAN B, SUN L, et al. Adding MgO nanoparticles to hydroxyapatite–PLLA nanocomposites for improved bone tissue engineering applications. Acta Biomater. 2015;14:175-184.
[40] BARNES D, JOHNSON S, SNELL R, et al. Using scratch testing to measure the adhesion strength of calcium phosphate coatings applied to poly (carbonate urethane) substrates. J Mech Behav Biomed Mater. 2012;6: 128-138.
[41] CAI Y, LI C, WU D, et al. Highly active MgO nanoparticles for simultaneous bacterial inactivation and heavy metal removal from aqueous solution. Chem Eng J. 2017;312:158-166.
[42] HE Y, INGUDAM S, REED S, et al. Study on the mechanism of antibacterial action of magnesium oxide nanoparticles against foodborne pathogens. J Nanobiotechnol. 2016;14:1-9.
[43] NAKAMURA T, NAGURO I, ICHIJO H. Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochim Biophys Acta Gen Subj. 2019;1863(9):1398-1409.
[44] BUCKLEY RRE, MORAN CG, APIVATTHAKAKUL T.骨折治疗的AO原则[M].危杰,刘璠,吴新宝,等译.3版.上海:上海科学技术出版社,2019:14-15.
[45] LIANG L, HUANG Q, WU H, et al. Stimulation of in vitro and in vivo osteogenesis by Ti-Mg alloys with the sustained-release function of magnesium ions. Colloids Surf B Biointerfaces. 2021;197:111360.
[46] WANG Y, LIANG W, LIU X, et al. Osteogenesis and degradation behavior of magnesium alloy plate in vivo. Eur J Inflamm. 2021;19: 20587392211034078.
[47] POURDANESH F, JEBALI A, HEKMATIMOGHADDAM S, et al. In vitro and in vivo evaluation of a new nanocomposite, containing high density polyethylene, tricalcium phosphate, hydroxyapatite, and magnesium oxide nanoparticles. Mater Sci Eng C Mater Biol Appl. 2014;40:382-388.
[48] JANNING C, WILLBOLD E, VOGT C, et al. Magnesium hydroxide temporarily enhancing osteoblast activity and decreasing the osteoclast number in peri-implant bone remodelling. Acta Biomater. 2010;6(5): 1861-1868.
[49] WITTE F, KAESE V, HAFERKAMP H, et al. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials. 2005;26(17):3557-3563. |