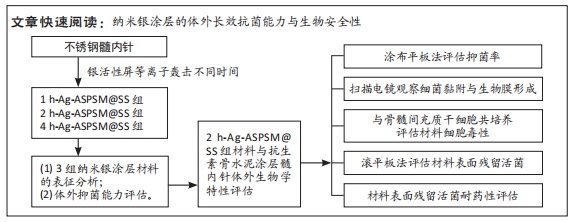
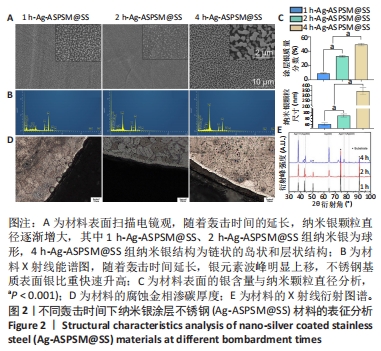
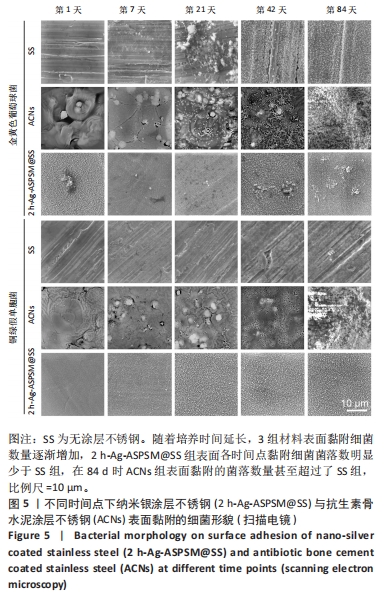
[1] ZHANG L, YANG Y, XIONG YH, et al. Infection-responsive long-term antibacterial bone plates for open fracture therapy. Bioact Mater. 2023; 25:1-25.
[2] TANWAR YS, FERREIRA N. The role of bioactive glass in the management of chronic osteomyelitis: a systematic review of literature and current evidence. Infect Dis (Lond). 2020;52(4):219-226.
[3] LI G, LV K, CHENG Q, et al. Enhanced Bacterial-Infected Wound Healing by Nitric Oxide-Releasing Topological Supramolecular Nanocarriers with Self-Optimized Cooperative Multi-Point Anchoring. Adv Sci (Weinh). 2023;10(11):e2206959.
[4] UDDIN TM, CHAKRABORTY AJ, KHUSRO A, et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J Infect Public Health. 2021;14(12):1750-1766.
[5] PULINGAM T, PARUMASIVAM T, GAZZALI AM, et al. Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur J Pharm Sci. 2022;170:106103.
[6] MASTERS EA, RICCIARDI BF, BENTLEY KLDM, et al. Skeletal infections: microbial pathogenesis, immunity and clinical management. Nat Rev Microbiol. 2022;20(7):385-400.
[7] CHENG X, PEI X, XIE W, et al. pH-Triggered Size-Tunable Silver Nanoparticles: Targeted Aggregation for Effective Bacterial Infection Therapy. Small. 2022;18(22):e2200915.
[8] FERRERES G, IVANOVA K, TORRENT-BURGUéS J, et al. Multimodal silver-chitosan-acylase nanoparticles inhibit bacterial growth and biofilm formation by Gram-negative Pseudomonas aeruginosa bacterium. J Colloid Interface Sci. 2023;646:576-586.
[9] WANG G, JIN W, QASIM AM, et al. Antibacterial effects of titanium embedded with silver nanoparticles based on electron-transfer-induced reactive oxygen species. Biomaterials. 2017;124:25-34.
[10] QIN H, CAO H, ZHAO Y, et al. Antimicrobial and osteogenic properties of silver-ion-implanted stainless steel.ACS Appl Mater Interfaces. 2015;7(20):10785-10794.
[11] MOUSAVI SM, HASHEMI SA, GHASEMI Y, et al. Green synthesis of silver nanoparticles toward bio and medical applications: review study. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S855-S872.
[12] YOUNAS W, KHAN FU, ZAMAN M, et al. Toxicity of synthesized silver nanoparticles in a widespread fish: A comparison between green and chemical. Sci Total Environ. 2022;845:157366.
[13] JI X, LI X, DONG Y, et al. Synthesis and in-vitro antibacterial properties of a functionally graded Ag impregnated composite surface. Mater Sci Eng C Mater Biol Appl. 2019;99:150-158.
[14] DONG Y, LI X, TIAN L, et al. Towards long-lasting antibacterial stainless steel surfaces by combining double glow plasma silvering with active screen plasma nitriding. Acta Biomater. 2011;7(1):447-457.
[15] KVRYAN A, EFAW CM, HIGGINBOTHAM KA, et al. Corrosion Initiation and Propagation on Carburized Martensitic Stainless Steel Surfaces Studied via Advanced Scanning Probe Microscopy. Materials (Basel). 2019;12(6):940.
[16] MENSAH LM, LOVE BJ. A meta-analysis of bone cement mediated antibiotic release: Overkill, but a viable approach to eradicate osteomyelitis and other infections tied to open procedures. Mater Sci Eng C Mater Biol Appl. 2021;123:111999.
[17] STRACY M, SNITSER O, YELIN I, et al. Minimizing treatment-induced emergence of antibiotic resistance in bacterial infections. Science. 2022;375(6583):889-894.
[18] SLANE J, GIETMAN B, SQUIRE M. Antibiotic elution from acrylic bone cement loaded with high doses of tobramycin and vancomycin. J Orthop Res. 2018;36(4):1078-1085.
[19] JUNG KH, LEE CC, KIM TH, et al. Does spiked tibial cement spacer reduce spacer-related problems in two-stage revision total knee arthroplasty for infection? Int Orthop. 2022;46(9):2009-2017.
[20] FEI Z, ZHANG Z, WANG Y, et al. Comparing the Efficacy of Articulating Spacers in Two-Stage Revision for Periprosthetic Joint Infection Following Total Knee Arthroplasty: All-Cement Spacers vs Sterilized Replanted Metal-Polyethylene Spacers. Int J Gen Med. 2022;15: 3293-3301.
[21] WANG G, LUO W, ZHOU Y, et al. Custom-Made Antibiotic Cement-Coated Nail for the Treatment of Infected Bone Defect. Biomed Res Int. 2021;2021:6693906.
[22] STREULI JC, EXNER GU, REIZE CL, et al. In vitro inhibition of coagulase-negative staphylococci by vancomycin/aminoglycoside-loaded cement spacers. Infection. 2006;34(2):81-86.
[23] BOELCH SP, JORDAN MC, ARNHOLDT J, et al. Antibiotic elution and compressive strength of gentamicin/vancomycin loaded bone cements are considerably influenced by immersion fluid volume. J Mater Sci Mater Med. 2019;30(2):29.
[24] BERBERICH C, SANZ-RUIZ P. Risk assessment of antibiotic resistance development by antibiotic-loaded bone cements: is it a clinical concern? EFORT Open Rev. 2019;4(10):576-584.
[25] VAN VUGT TAG, ARTS JJ, GEURTS JAP. Antibiotic-Loaded Polymethylmethacrylate Beads and Spacers in Treatment of Orthopedic Infections and the Role of Biofilm Formation. Front Microbiol. 2019; 10:1626.
[26] MENDEZ-PFEIFFER P, BALLESTEROS-MONRREAL MG, GAONA-OCHOA J, et al. Biosynthesis of Silver Nanoparticles Using Seasonal Samples of Sonoran Desert Propolis: Evaluation of Its Antibacterial Activity against Clinical Isolates of Multi-Drug Resistant Bacteria. Pharmaceutics. 2022; 14(9):1853.
[27] MENICHETTI A, MAVRIDI-PRINTEZI A, MORDINI D, et al. Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. J Funct Biomater. 2023;14(5):244.
[28] WAKSHLAK RB, PEDAHZUR R, AVNIR D. Antibacterial activity of silver-killed bacteria: the “zombies” effect. Sci Rep. 2015;5:9555.
[29] BOONRUANG C, SANUMANG W. Effect of nano-grain carbide formation on electrochemical behavior of 316L stainless steel. Sci Rep. 2021;11(1):12602.
[30] CAO H, QIAO Y, LIU X, et al. Electron storage mediated dark antibacterial action of bound silver nanoparticles: smaller is not always better. Acta Biomater. 2013;9(2):5100-5110.
[31] CALABRESE G, PETRALIA S, FRANCO D, et al. A new Ag-nanostructured hydroxyapatite porous scaffold: Antibacterial effect and cytotoxicity study. Mater Sci Eng C Mater Biol Appl. 2021;118:111394.
[32] LASHIN I, FOUDA A, GOBOURI A A, et al. Antimicrobial and In Vitro Cytotoxic Efficacy of Biogenic Silver Nanoparticles (Ag-NPs) Fabricated by Callus Extract of Solanum incanum L. Biomolecules. 2021;11(3):341.
[33] SOARES T, RIBEIRO D, PROENçA C, et al. Size-dependent cytotoxicity of silver nanoparticles in human neutrophils assessed by multiple analytical approaches. Life Sci. 2016;145:247-254.
[34] PARK MV, NEIGH AM, VERMEULEN JP, et al. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials. 2011;32(36):9810-9817.
[35] KOSE O, MANTECCA P, COSTA A, et al. Putative adverse outcome pathways for silver nanoparticle toxicity on mammalian male reproductive system: a literature review. Part Fibre Toxicol. 2023;20(1):1.
[36] QIN H, CAO H, ZHAO Y, et al. In vitro and in vivo anti-biofilm effects of silver nanoparticles immobilized on titanium. Biomaterials. 2014;35(33): 9114-9125. |