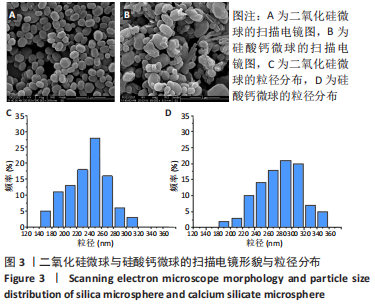
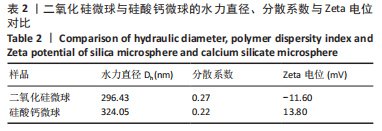
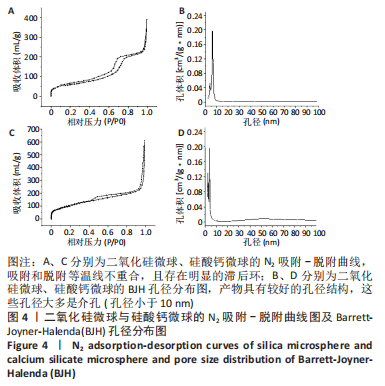
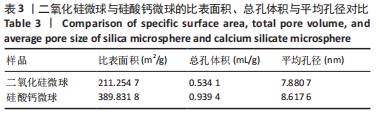
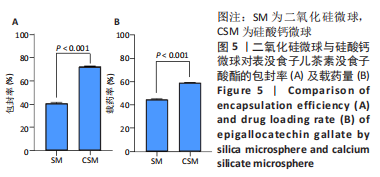
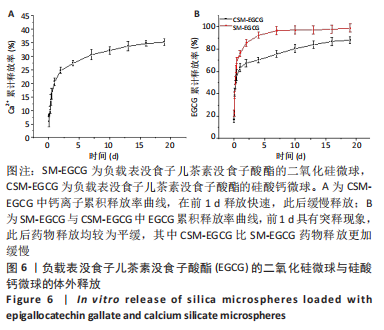
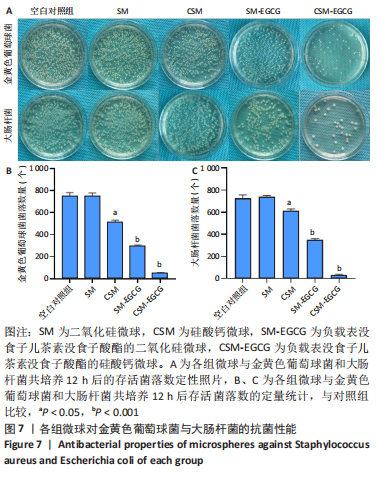
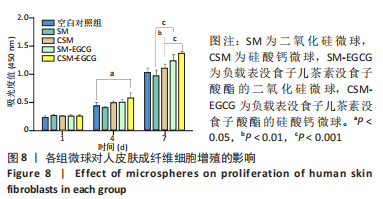
[1] KHAN N, MUKHTAR H. Tea polyphenols in promotion of human health. Nutrients. 2018;11(1):39.
[2] XING L, ZHANG H, QI R, et al. Recent advances in the understanding of the health benefits and molecular mechanisms associated with green tea polyphenols. J Agric Food Chem. 2019;67(4):1029-1043.
[3] CARSON M, KEPPLER JK, BRACKMAN G, et al. Whey protein complexes with green tea polyphenols: antimicrobial, osteoblast-stimulatory, and antioxidant activities. Cells Tissues Organs. 2018;206(1-2):106-118.
[4] YANG Y, ZHANG T. Antimicrobial activities of tea polyphenol on phytopathogens: A review. Molecules. 2019;24(4):816.
[5] TROBOS M, FIRDAUS R, SVENSSON MALCHAU K, et al. Genomics of staphylococcus aureus and staphylococcus epidermidis from periprosthetic joint infections and correlation to clinical outcome. Microbiol Spectr. 2022;10(4):e0218121.
[6] MIKLASINSKA-MAJDANIK M, KEPA M, WOJTYCZKA RD, et al. Phenolic compounds diminish antibiotic resistance of staphylococcus aureus clinical strains. Int J Environ Res Public Health. 2018;15(10):2321.
[7] IGNASIMUTHU K, PRAKASH R, MURTHY PS, et al. Enhanced bioaccessibility of green tea polyphenols and lipophilic activity of EGCG octaacetate on gram-negative bacteria. Lwt. 2019;105:103-109.
[8] XU X, DAI Z, ZHANG Z, et al. Fabrication of oral nanovesicle in-situ gel based on epigallocatechin gallate phospholipid complex: Application in dental anti-caries. Eur J Pharmacol. 2021;897:173951.
[9] TOUYA N, WASHIO A, KITAMURA C, et al. In Vivo Application of Silica-Derived Inks for Bone Tissue Engineering: A 10-Year Systematic Review. Bioengineering (Basel). 2022;9(8):388.
[10] JI G, XIONG G, PENG X, et al. Quantitative evaluation of carbon fiber dispersion in amorphous calcium silicate hydrate-based contact-hardening composites. Molecules. 2021;26(3):726.
[11] WANG S, JIANG L, MENG S, et al. Hollow mesoporous silica nanoparticles-loaded ion-crosslinked bilayer films with excellent mechanical properties and high bioavailability for buccal delivery. Int J Pharm. 2022;624:122056.
[12] WANG X, LI X, LIANG X, et al. ROS-responsive capsules engineered from green tea polyphenol-metal networks for anticancer drug delivery. J Mater Chem B. 2018;6(7):1000-1010.
[13] ZHANG X, LI Z, YANG P, et al. Polyphenol scaffolds in tissue engineering. Mater Horiz. 2021;8(1):145-167.
[14] RANA A, SAMTIYA M, DHEWA T, et al. Health benefits of polyphenols: A concise review. J Food Biochem. 2022;46(10):e14264.
[15] LIN X, ZHANG H, LI S, et al. Polyphenol-driving assembly for constructing chitin-polyphenol-metal hydrogel as wound dressing. Carbohydr Polym. 2022;290: 119444.
[16] NIU L, LI Z, FAN W, et al. Nano-Strategies for enhancing the bioavailability of tea polyphenols: Preparation, applications, and challenges. Foods (Basel, Switzerland). 2022;11(3):387.
[17] YIN C, CHENG L, ZHANG X, et al. Nanotechnology improves delivery efficiency and bioavailability of tea polyphenols. J Food Biochem. 2020;44(9):e13380.
[18] WU S, YANG X, LUO F, et al. Biosynthesis of flower-shaped Au nanoclusters with EGCG and their application for drug delivery. J Nanobiotechnol. 2018;16(1):90.
[19] REN X, ZHANG P, CHEN Z. Dialysis preparation of smart redox and acidity dual responsive tea polyphenol functionalized calcium phosphate panospheres as anticancer drug carriers. Molecules (Basel, Switzerland). 2020;25(5):1221.
[20] ZHANG S, CHU Z, YIN C, et al. Controllable drug release and simultaneously carrier decomposition of SiO2-drug composite nanoparticles. J Am Chem Soc. 2013;135(15):5709-5716.
[21] 李兴平,肖东琴,赵桥,等.钛表面载铜抗菌功能膜的制备及性能[J].中国组织工程研究,2021,25(4):553.
[22] REN Y, WANG FY, CHEN ZJ, et al. Antibacterial and anti-inflammatory ultrahigh molecular weight polyethylene/tea polyphenol blends for artificial joint applications. J Mater Chem B. 2020;8(45):10428-10438.
[23] 叶晴,刘毅,陈金鹏,等.绿茶化学成分及药理作用研究进展[J].药物评价研究,2021,44(12): 2711-2719.
[24] Hetrick EM, Schoenfisch MH. Reducing implant-related infections: Active release strategies. Chem Soc Rev. 2006;35:780-789.
[25] YIN X, HUANG S, XU S, et al. Preparation of pro-angiogenic, antibacterial and EGCG-modified ZnO quantum dots for treating bacterial infected wound of diabetic rats. Biomater Adv. 2022;133:112638.
[26] KWON YS, KIM HJ, HWANG YC, et al. Effects of epigallocatechin gallate, an antibacterial cross-linking agent, on proliferation and differentiation of human dental pulp cells cultured in collagen scaffolds. J Endod. 2017;43(2):289-296.
[27] LIN MC, CHEN CC, WU IT, et al. Enhanced antibacterial activity of calcium silicate-based hybrid cements for bone repair. Mater Sci Eng C Mater Biol Appl. 2020;110:110727.
[28] HUANG TW, LU HT, HO YC, et al. A smart and active film with tunable drug release and color change abilities for detection and inhibition of bacterial growth. Mater Sci Eng C Mater Biol Appl. 2021;118:111396.
[29] FERREIRA GC, PINHEIRO LS, NUNES JS, et al. Evaluation of the biological and physicochemical properties of calcium silicate-based and epoxy resin-based root canal sealers. J Biomed Mater Res B Appl Biomater. 2022;110(6):1344-1353.
[30] LEONG DJ, CHOUDHURY M, HANSTEIN R, et al. Correction to: green tea polyphenol treatment is chondroprotective, anti-inflammatory and palliative in a mouse posttraumatic osteoarthritis model. Arthritis Res Ther. 2019;21(1):1.
[31] WANG J, LEI J, HU Y, et al. Calcium silicate whiskers-enforced poly(ether-ether-ketone) composites with improved mechanical properties and piological activities for bearing bone reconstruction. Macromol Biosci. 2022;e2200321. doi: 10.1002/mabi.202200321.
[32] MOHAMED ABUDHAHIR K, MURUGESAN R, VIJAYASHREE R, et al. Metal doped calcium silicate biomaterial for skin tissue regeneration in vitro. J Biomater Appl. 2021;36(1):140-151.
[33] LEE BS, LEE CC, LIN HP, et al. A functional chitosan membrane with grafted epigallocatechin-3-gallate and lovastatin enhances periodontal tissue regeneration in dogs. Carbohydr polym. 2016;151:790-802. |