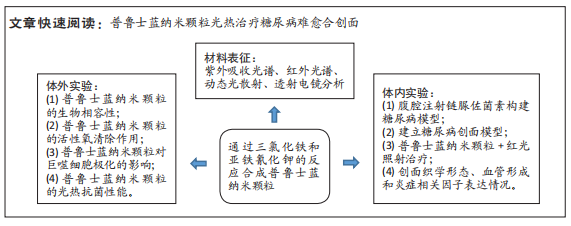
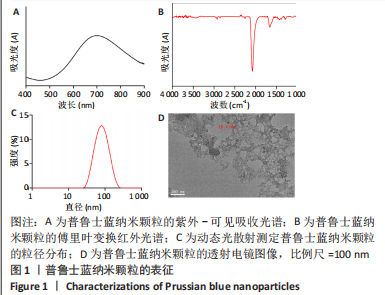
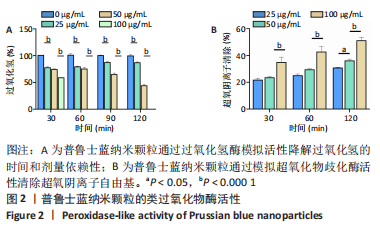
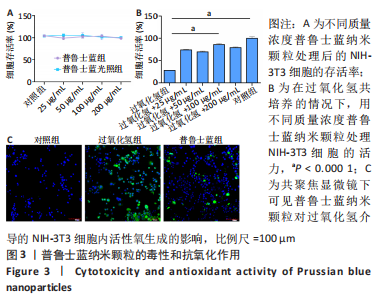
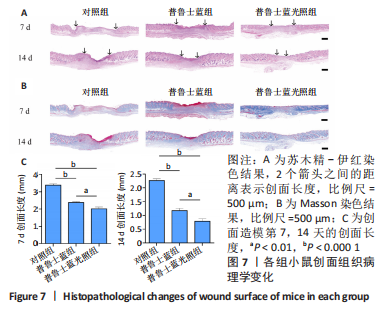
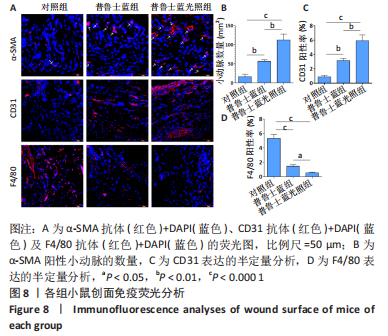
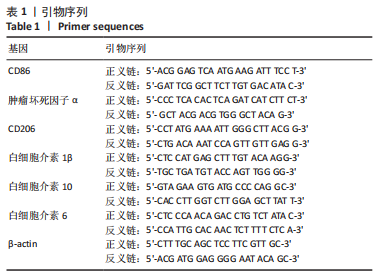
[1] BURGESS JL, WYANT WA, ABDO ABUJAMRA B, et al. Diabetic Wound-Healing Science. Medicina (Kaunas). 2021;57(10):1072.
[2] DUNNILL C, PATTON T, BRENNAN J, et al. Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int Wound J. 2017;14(1):89-96.
[3] DENG L, DU C, SONG P, et al. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid Med Cell Longev. 2021;2021:8852759.
[4] KUNKEMOELLER B, KYRIAKIDES TR. Redox Signaling in Diabetic Wound Healing Regulates Extracellular Matrix Deposition. Antioxid Redox Signal. 2017;27(12): 823-838.
[5] CHANG M, NGUYEN TT. Strategy for Treatment of Infected Diabetic Foot Ulcers. Acc Chem Res. 2021;54(5):1080-1093.
[6] PATEL S, SRIVASTAVA S, SINGH MR, et al. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed Pharmacother. 2019;112:108615.
[7] OYEBODE O, HOURELD NN, ABRAHAMSE H. Photobiomodulation in diabetic wound healing: A review of red and near-infrared wavelength applications. Cell Biochem Funct. 2021;39(5):596-612.
[8] EZHILARASU H, VISHALLI D, DHEEN ST, et al. Nanoparticle-Based Therapeutic Approach for Diabetic Wound Healing. Nanomaterials (Basel). 2020;10(6):1234.
[9] WANG F, ZHANG W, LI H, et al. How Effective are Nano-Based Dressings in Diabetic Wound Healing? A Comprehensive Review of Literature. Int J Nanomedicine. 2022;17:2097-2119.
[10] BAI Q, HAN K, DONG K, et al. Potential Applications of Nanomaterials and Technology for Diabetic Wound Healing. Int J Nanomedicine. 2020;15:9717-9743.
[11] SABAREES G, VELMURUGAN V, TAMILARASI GP, et al. Recent Advances in Silver Nanoparticles Containing Nanofibers for Chronic Wound Management. Polymers (Basel). 2022;14(19):3994.
[12] MASOOD N, AHMED R, TARIQ M, et al. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int J Pharm. 2019;559:23-36.
[13] ZHAO X, CHANG L, HU Y, et al. Preparation of Photocatalytic and Antibacterial MOF Nanozyme Used for Infected Diabetic Wound Healing. ACS Appl Mater Interfaces. 2022;14(16):18194-18208.
[14] CHEN YH, RAO ZF, LIU YJ, et al. Multifunctional Injectable Hydrogel Loaded with Cerium-Containing Bioactive Glass Nanoparticles for Diabetic Wound Healing. Biomolecules. 2021; 11(5):702.
[15] LI S, WANG X, CHEN J, et al. Calcium ion cross-linked sodium alginate hydrogels containing deferoxamine and copper nanoparticles for diabetic wound healing. Int J Biol Macromol. 2022;202:657-670.
[16] GAN J, LIU C, LI H, et al. Accelerated wound healing in diabetes by reprogramming the macrophages with particle-induced clustering of the mannose receptors. Biomaterials. 2019; 219:119340.
[17] 吴海能,耿康,王静,等.富血小板纤维蛋白联合姜黄素纳米颗粒水凝胶促糖尿病小鼠创面愈合[J].中国组织工程研究,2022,26(27):4300-4307.
[18] WANG Y, PANG X, WANG J, et al. Magnetically-targeted and near infrared fluorescence/magnetic resonance/photoacoustic imaging-guided combinational anti-tumor phototherapy based on polydopamine-capped magnetic Prussian blue nanoparticles. J Mater Chem B. 2018;6(16):2460-2473.
[19] LU L, ZHANG C, ZOU B, et al. Hollow Prussian Blue Nanospheres for Photothermal/Chemo-Synergistic Therapy. Int J Nanomedicine. 2020;15: 5165-5177.
[20] WANG S, ZENG N, ZHANG Q, et al. Nanozyme Hydrogels for Self-Augmented Sonodynamic/Photothermal Combination Therapy. Front Oncol. 2022;12:888855.
[21] XU M, GAO H, JI Q, et al. Construction multifunctional nanozyme for synergistic catalytic therapy and phototherapy based on controllable performance. J Colloid Interface Sci. 2022; 609:364-374.
[22] MAAOUI H, JIJIE R, PAN GH, et al. A 980nm driven photothermal ablation of virulent and antibiotic resistant Gram-positive and Gram-negative bacteria strains using Prussian blue nanoparticles. J Colloid Interface Sci. 2016;480:63-68.
[23] BORZENKOV M, CHIRICO G, PALLAVICINI P, et al. Nanocomposite Sprayed Films with Photo-Thermal Properties for Remote Bacteria Eradication. Nanomaterials (Basel). 2020;10(4):786.
[24] LI Y, YU P, WEN J, et al. Nanozyme‐Based Stretchable Hydrogel of Low Hysteresis with Antibacterial and Antioxidant Dual Functions for Closely Fitting and Wound Healing in Movable Parts. Adv Funct Mater. 2021;32(13):2110720.
[25] ESTELRICH J, BUSQUETS MA. Prussian Blue: A Nanozyme with Versatile Catalytic Properties. Int J Mol Sci. 2021;22(11):5993.
[26] 王青,刘学,孙亚娟,等.负载普鲁士蓝的CoOOH纳米片用于抗氧化活性检测的研究[J].现代化工,2023,43(2):234-238.
[27] WANG Y, LIANG Z, LIANG Z, et al. Advancements of Prussian blue-based nanoplatforms in biomedical fields: Progress and perspectives. J Control Release. 2022;351:752-778.
[28] SAHU A, JEON J, LEE MS, et al. Antioxidant and anti-inflammatory activities of Prussian blue nanozyme promotes full-thickness skin wound healing. Mater Sci Eng C Mater Biol Appl. 2021;119:111596.
[29] GANTWERKER EA, HOM DB. Skin: histology and physiology of wound healing. Clin Plast Surg. 2012;39(1):85-97.
[30] VELNAR T, BAILEY T, SMRKOLJ V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37(5):1528-1542.
[31] MARTIN P, NUNAN R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173(2):370-378.
[32] BURANASIN P, MIZUTANI K, IWASAKI K, et al. High glucose-induced oxidative stress impairs proliferation and migration of human gingival fibroblasts. PLoS One. 2018;13(8):e0201855.
[33] GONG F, ZHANG Y, CHENG S, et al. Inhibition of TGFbeta1/Smad pathway by NF-kappaB induces inflammation leading to poor wound healing in high glucose. Cells Dev. 2022;172:203814.
[34] QIN W, WU Y, LIU J, et al. A Comprehensive Review of the Application of Nanoparticles in Diabetic Wound Healing: Therapeutic Potential and Future Perspectives. Int J Nanomedicine. 2022;17:6007-6029.
[35] SAYED FA, EISSA NG, SHEN Y, et al. Morphologic design of nanostructures for enhanced antimicrobial activity. J Nanobiotechnology. 2022;20(1):536.
[36] SAHOO BM, BANIK BK, BORAH P, et al. Reactive Oxygen Species (ROS): Key Components in Cancer Therapies. Anticancer Agents Med Chem. 2022; 22(2):215-222.
[37] NIKITINA VN, ZAVOLSKOVA MD, KARYAKIN AA. Protein-sized nanozymes <<artificial peroxidase>> based on template catalytic synthesis of Prussian Blue. Bioelectrochemistry. 2023;149:108275.
[38] LI Z, GUO X, QIN J, et al. Size-effect on the intracellular antioxidative activity of Prussian blue nanoparticles investigated by atomic force microscopy. Anal Chim Acta. 2022;1227:340321.
[39] AITCHESON SM, FRENTIU FD, HURN SE, et al. Skin Wound Healing: Normal Macrophage Function and Macrophage Dysfunction in Diabetic Wounds. Molecules. 2021;26(16):4917.
[40] RAZIYEVA K, KIM Y, ZHARKINBEKOV Z, et al. Immunology of Acute and Chronic Wound Healing. Biomolecules. 2021;11(5):700.
[41] LOUISELLE AE, NIEMIEC SM, ZGHEIB C, et al. Macrophage polarization and diabetic wound healing. Transl Res. 2021;236:109-116.
[42] LI D, LIU M, LI W, et al. Synthesis of Prussian Blue Nanoparticles and Their Antibacterial, Antiinflammation and Antitumor Applications. Pharmaceuticals (Basel). 2022;15(7):769.
[43] SAHU A, JEON J, LEE MS, et al. Nanozyme Impregnated Mesenchymal Stem Cells for Hepatic Ischemia-Reperfusion Injury Alleviation. ACS Appl Mater Interfaces. 2021;13(22):25649-25662.
[44] TONG C, ZHONG X, YANG Y, et al. PB@PDA@Ag nanosystem for synergistically eradicating MRSA and accelerating diabetic wound healing assisted with laser irradiation. Biomaterials. 2020;243:119936.
[45] TAN L, LI J, LIU X, et al. Rapid Biofilm Eradication on Bone Implants Using Red Phosphorus and Near-Infrared Light. Adv Mater. 2018;30(31):e1801808.
[46] SUN J, SONG L, FAN Y, et al. Synergistic Photodynamic and Photothermal Antibacterial Nanocomposite Membrane Triggered by Single NIR Light Source. ACS Appl Mater Interfaces. 2019;11(30):26581-26589.
[47] WANG X, SUN X, BU T, et al. Construction of a photothermal hydrogel platform with two-dimensional PEG@zirconium-ferrocene MOF nanozymes for rapid tissue repair of bacteria-infected wounds. Acta Biomater. 2021; 135:342-355.
|