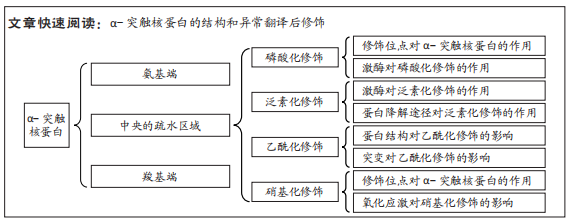
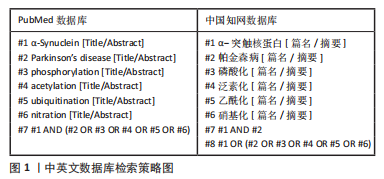
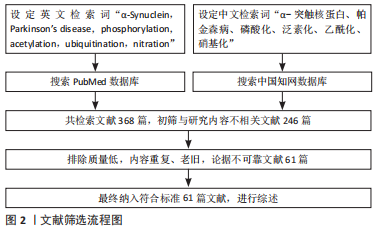
[1] GOEDERT M, JAKES R, SPILLANTINI MG. The Synucleinopathies: twenty years on. J Parkinsons Dis. 2017;7(s1):S51-S69.
[2] WEINTRAUB D, MAMIKONYAN E. The neuropsychiatry of Parkinson disease: a perfect storm. Am J Geriatr Psychiatry. 2019;27(9):998-1018.
[3] BLESA J, FOFFANI G, DEHAY B, et al. Motor and non-motor circuit disturbances in early Parkinson disease: which happens first? Nat Rev Neurosci. 2022;23(2):115-128.
[4] POYMEROPOULOS MH, LAVEDAN C, LEROY E, et al. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045-2047.
[5] WON SJ, FONG R, BUTLER N, et al. Neuronal oxidative stress promotes α-synuclein aggregation in vivo. Antioxidants (Basel). 2022;11(12):2466.
[6] STEFANIS L, EMMANOILIDOU E, PANTAZOPOULOU M, et al. How is alpha‐synuclein cleared from the cell? J Neurochem. 2019;150:577-590.
[7] YOO H, LEE J, KIM B, et al. Role of post-translational modifications on the alpha-synuclein aggregation-related pathogenesis of Parkinson’s disease. BMB Rep. 2022; 55(7):323-335.
[8] HENDERSON MX, TROJANOWSKI JQ, LEE MY. α-synuclein pathology in Parkinson’s disease and related α-synucleinopathies. Neurosci Lett. 2019;709:134316.
[9] SARCHIONE A, MARCHAND A, TAYMANS JM, et al. Alpha-synuclein and lipids: the elephant in the room? Cells. 2021;10(9):2452.
[10] ZHAO K, LI Y, LIU Z, et al. Parkinson’s disease associated mutation E46K of α-synuclein triggers the formation of a distinct fibril structure. Nat Commun. 2020;11(1):2643.
[11] BELL R, CASTELLANA-CRUZ M, NENE A, et al. Effects of n-terminal acetylation on the aggregation of disease-related α-synuclein variants. J Mol Biol. 2023;435(1):167825.
[12] HOLEC SAM, LEE J, OEHLER A, et al. The E46K mutation modulates α-synuclein prion replication in transgenic mice. PLoS Pathog. 2022;18(12):e1010956.
[13] MCGLINCHEY RP, NI X, SHADISH JA, et al. The N terminus of α-synuclein dictates fibril formation. Proc Natl Acad Sci U S A. 2021;118(35):e2023487118.
[14] KAPASI A, BROSCH JR, NUDELMAN KN, et al. A novel SNCA E83Q mutation in a case of dementia with Lewy bodies and atypical frontotemporal lobar degeneration. Neuropathology. 2020;40(6):620-626.
[15] KUMAR ST, MAHUL-MELLIER AL, HEGDE RN, et al. A NAC domain mutation (E83Q) unlocks the pathogenicity of human alpha-synuclein and recapitulates its pathological diversity. Sci Adv. 2022;8(17):eabn0044.
[16] NASSTROM T, DAHLBERG T, MALYSHEV D, et al. Synthetic NAC 71-82 peptides designed to produce fibrils with different protofilament interface contacts. Int J Mol Sci. 2021;22(17):9334.
[17] KACHAPPILLY N, SRIVASTAVA J, SWAIN BP, et al. Interaction of alpha-synuclein with lipids. Methods Cell Biol. 2022;169:43-66.
[18] DASARI AKR, KAYED R, WI S, et al. Tau interacts with the C-terminal region of α-synuclein, promoting formation of toxic aggregates with distinct molecular conformations. Biochemistry. 2019;58(25):2814 -2821.
[19] VAN DER WATEREN IM, KNOELES TPJ, BUELL AK, et al. C-terminal truncation of α-synuclein promotes amyloid fibril amplification at physiological pH. Chem Sci. 2018;9(25):5506-5516.
[20] ESTAUN-PANZANO J, AROTCARENA ML, BEZARD E. Monitoring α-synuclein aggregation. Neurobiol Dis. 2023;176:105966.
[21] 李冬青,秦晓红,米立志.α-突触核蛋白的结构生物学研究[J].中国生物化学与分子生物学报,2023,39(4):531-544.
[22] 李蕾,邢昊,胡家,等.蛋白质磷酸化位点富集与鉴定方法的建立与应用[J].分析试验室,2015,34(11):1348-1352.
[23] UBBIALI D, FRATINI M, PIERSIMONI L, et al. Direct observation of “elongated” conformational states in α-synuclein upon liquid-liquid phase separation. Angew Chem Int Ed Engl. 2022;61(46):e202205726.
[24] KAWAHATA I, FINKELSTEIN DI, FUKUNAGA K. Pathogenic impact of α-synuclein phosphorylation and its kinases in α-synucleinopathies. Int J Mol Sci. 2022;23(11): 6216.
[25] ANDERSON JP, WALKER DE, GODSTEIN JM, et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281(40):29739-29752.
[26] VISANJI NP, WISLER-GENDEBIEN S, OSCHIPOK LW, et al. Withdrawal: effect of Ser-129 phosphorylation on interaction of α-synuclein with synaptic and cellular membranes [retraction of: J Biol Chem. 2011;286(41):35863-73]. J Biol Chem. 2020;295(39):13695.
[27] GABRILIYAN L, LIANG H, MINALYAN A, et al. Behavioral deficits and brain α-synuclein and phosphorylated serine-129 α-synuclein in male and female mice overexpressing human α-synuclein. J Alzheimers Dis. 2021;79(2):875-893.
[28] DING J, WANG Y, HUANG J, et al. Role of alpha-synuclein phosphorylation at Serine 129 in methamphetamine-induced neurotoxicity in vitro and in vivo. Neuroreport. 2020;31(11):787-797.
[29] GHANEM SIMONA S, MAJBOUR NOUR K, VAIKATH NISHANT N, et al. α-Synuclein phosphorylation at serine 129 occurs after initial protein deposition and inhibits seeded fibril formation and toxicity. Proc Natl Acad Sci U S A. 2022;119: e2109617119.
[30] VUIDEL A, COUSIN L, WEYKOPF B, et al. High-content phenotyping of Parkinson’s disease patient stem cell-derived midbrain dopaminergic neurons using machine learning classification. Stem Cell Reports. 2022;17(10):2349-2364.
[31] CARIULO C, MARTUFI P, VERANI M, et al. Phospho-S129 alpha-synuclein is present in human plasma but not in cerebrospinal fluid as determined by an ultrasensitive immunoassay. Front Neurosci. 2019;13:889.
[32] ARLINGHAUS R, IBA M, MASLIASH E, et al. Specific detection of physiological s129 phosphorylated α-synuclein in tissue using proximity ligation assay. J Parkinsons Dis. 2023;13(2):255-270.
[33] WU W, SUNG CC, YU P, et al. Correction: S-nitrosylation of G protein-coupled receptor kinase 6 and Casein kinase 2 alpha modulates their kinase activity toward alpha-synuclein phosphorylation in an animal model of Parkinson’s disease. PLoS One. 2020;15(6):e0235296.
[34] SANO K, IWASAKI Y, YAMASHITA Y, et al. Tyrosine 136 phosphorylation of α-synuclein aggregates in the Lewy body dementia brain: involvement of serine 129 phosphorylation by casein kinase 2. Acta Neuropathol Commun. 2021;9(1):182.
[35] KEDARITI M, FRATTINI E, BADEN P, et al. LRRK2 kinase activity regulates GCase level and enzymatic activity differently depending on cell type in Parkinson’s disease. NP J Parkinsons Dis. 2022;8(1):92.
[36] VERBINNEN I, VANEYNDE P, REYNHOUT S, et al. Protein Phosphatase 2A (PP2A) mutations in brain function, development, and neurologic disease. Biochem Soc Trans. 2021;49(4):1567-1588.
[37] RUVOLO PP, DENG X, ITO T, et al. Ceramide induces Bcl2 dephosphorylation via a mechanism involving mitochondrial PP2A. J Biol Chem. 1999;274(29):20296-20300.
[38] 安俊言,安思训,王鹏.帕金森病患者血清polo样激酶2活性升高促进α-突触核蛋白聚集[J].吉林医药学院学报,2019,40(3):171-174.
[39] TOMA-FUKAI S, SHIMIZU T. Structural diversity of ubiquitin E3 ligase. Molecules. 2021;26(21):6682.
[40] MOON SP, BALANA AT, GALESIC A, et al. Ubiquitination can change the structure of the α-synuclein amyloid fiber in a site selective fashion. J Org Chem. 2020;85(3): 1548-1555.
[41] MEIER F, ABEYWARDANA T, DHALL A, et al. Semisynthetic,site-specific ubiquitin modification of α-synuclein reveals differential effects on aggregation. J Am Chem Soc. 2012;134(12):5468-5471.
[42] WANG QJ, CHEN AD, ChEN HC, et al. Noncanonical roles of h α-syn (A53T) in the pathogenesis of parkinson’s disease: synaptic pathology and neuronal aging. Neural Plast. 2020(4):1-17.
[43] 王洪财.P75介导的泛素化修饰在帕金森病α-突触核蛋白聚集中的调控机制[D].滨州:滨州医学院,2020.
[44] BEHL T, KUMAR S, ALTHAFAR ZM, et al. Exploring the role of ubiquitin-proteasome system in Parkinson’s disease. Mol Neurobiol. 2022;59(7):4257-4273.
[45] QUINN PMJ, MOREIRA PI, AMBROSIO AF, et al. PINK1/PARKIN signalling in neurodegeneration and neuroinflammation. Acta Neuropathol Commun. 2020; 8(1):189.
[46] PARK SS, DO HA, PARK HB, et al. Deubiquitinating enzyme YOD1 deubiquitinates and destabilizes α-synuclein. Biochem Biophys Res Commun. 2023;645:124-131.
[47] XU L, BHATTACHARYA S, THOMPSON D. On the ubiquity of helical α-synuclein tetramers. Phys Chem Chem Phys. 2019;21(22):12036-12043.
[48] WATSON MD, LEE JC. N-terminal acetylation affects α-synuclein fibril polymorphism. Biochemistry. 2019;58(35):3630-3633.
[49] BELL R, THRUSH RJ, CASTELLANA-CRUZ M, et al. N-terminal acetylation of α-synuclein slows down its aggregation process and alters the morphology of the resulting aggregates. Biochemistry. 2022;61(17):1743-1756.
[50] 翟紫凝,吴琼,李从刚.乙酰化修饰抑制α-synuclein的纤维化聚集(英文)[J].波谱学杂志,2016,33(2):179-187.
[51] YANG X, WANG B, HOOP CL, et al. NMR unveils an N-terminal interaction interface on acetylated-α-synuclein monomers for recruitment to fibrils. Proc Natl Acad Sci U S A. 2021;118(18):e2017452118.
[52] KANG L, JANOWSKA MK, MORIARTY GM, et al. Mechanistic insight into the relationship between N-terminal acetylation of α-synuclein and fibril formation rates by NMR and fluorescence. PLoS One. 2013;8(9):e75018.
[53] TENG X, SHEVELEVA A, TUNA F, et al. Acetylation rather than H50Q mutation impacts the kinetics of Cu(II) binding to α-synuclein. Chemphyschem. 2021;22(23):2413-2419.
[54] XIE Y, LUO X, LI Y. DeepNitro: prediction of protein nitration and nitrosylation sites by deep learning. Genomics, Proteomics Bioinformatics. 2018;16:294-306
[55] QIAO HH, ZHU LN, WANG Y, et al. Implications of alpha-synuclein nitration at tyrosine 39 in methamphetamine-induced neurotoxicity in vitro and in vivo. Neural Regen Res. 2019;14(2):319-327.
[56] LIU Y, QIANG M, WEI Y, et al. A novel molecular mechanism for nitrated {alpha}-synuclein-induced cell death. J Mol Cell Biol. 2011;3(4):239-249.
[57] MUSGROVE RE, HELWIG M, BAE EJ, et al. Oxidative stress in vagal neurons promotes parkinsonian pathology and intercellular α-synuclein transfer. J Clin Invest. 2019; 129(9):3738-3753.
[58] STYKEL MG ,HUMPHRIES K, KIRBY MP, et al. Nitration of microtubules blocks axonal mitochondrial transport in a human pluripotent stem cell model of Parkinson’s disease. FASEB J. 2018;32:5350-5364.
[59] GIASSON BI, DUDA JE, MURRAY IVJ, et al. Oxidative damage linked to neurodegeneration by selective a-synuclein nitration in synucleinopathy lesions. Science. 2000;290(5493):985-989.
[60] SANTOS-LOBATO BL, BORTOLANZA M, PINHEIRO LC, et al. Levodopa-induced dyskinesias in Parkinson’s disease increase cerebrospinal fluid nitric oxide metabolites’ levels. J Neural Transm (Vienna). 2022;129:55-63.
[61] MA LY, GAO LY, Li X, et al. Nitrated alpha-synuclein in minor salivary gland biopsies in Parkinson’s disease. Neurosci Lett. 2019;704:45-49.
|