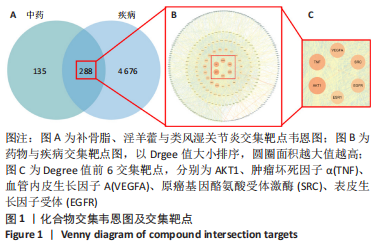
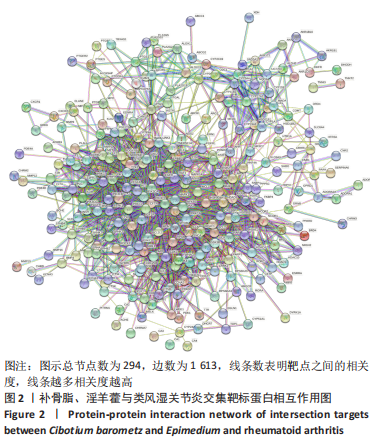
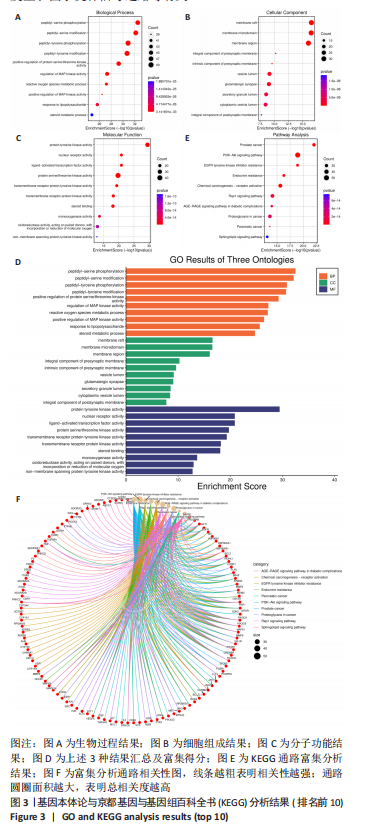
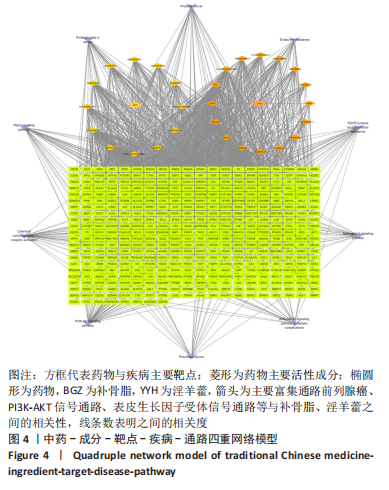
[1] TIAN X, WANG Q, LI M, et al. 2018 Chinese guidelines for the diagnosis and treatment of rheumatoid arthritis. Rheumatol Immunol Res. 2021;2:1-14.
[2] CHAURASIA N, SINGH A, SINGH IL, et al. Cognitive dysfunction in patients of rheumatoid arthritis. J Fam Med Prim Care. 2020;9:2219-2225.
[3] LASSERE MN, RAPPO J, PORTEK IJ, et al. How many life years are lost in patients with rheumatoid arthritis? Secular cause-specific and all-cause mortality in rheumatoid arthritis, and their predictors in a long-term Australian cohort study. Intern Med J. 2013;43(1):66-72.
[4] 童荣生.生物制剂治疗类风湿关节炎合理用药中国专家共识[J].中国新药杂志,2022,31(21):2174-2184.
[5] 《类风湿关节炎超药品说明书用药中国专家共识》制定专家组.类风湿关节炎超药品说明书用药中国专家共识(2022版)[J].中华医学杂志,2022,102(15): 1076-1085.
[6] 孙青,谢瑶,赵卫红,等.大剂量甲氨蝶呤治疗毒副作用系统分析[J].中国当代儿科杂志,2017,19(7):781-785.
[7] SMOLEN JS, LANDEWÉ RBM, BIJLSMA JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685-699.
[8] 顾娜沙. TNF-α抑制剂治疗类风湿关节炎患者用药现状的单中心观察研究[D].乌鲁木齐:新疆医科大学,2022.
[9] 王慧,王永福.RA生物制剂治疗[J].中国免疫学杂志,2017,33(12):1911-1916.
[10] 肖涟波,席智杰,程少丹,等.施杞从热毒痹论治急性期类风湿关节炎[J].上海中医药杂志,2017,51(12):1-4.
[11] 尹梦碟. 邱联群教授辨治类风湿关节炎的用药规律与相关文献研究[D].广州:广州中医药大学,2021.
[12] 杨静,梁江,唐东昕,等.国医大师刘尚义教授治疗类风湿关节炎的用药规律分析[J].风湿病与关节炎,2021,10(5):5-10.
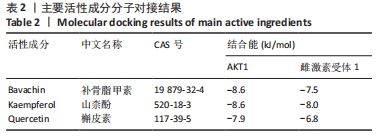
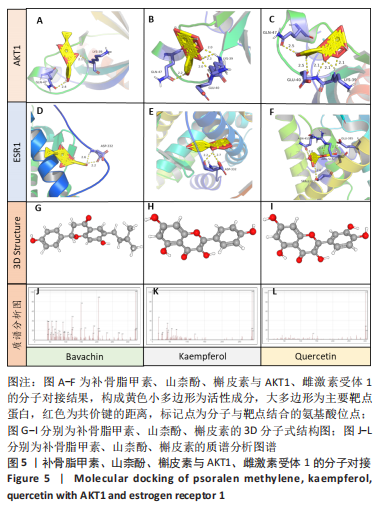
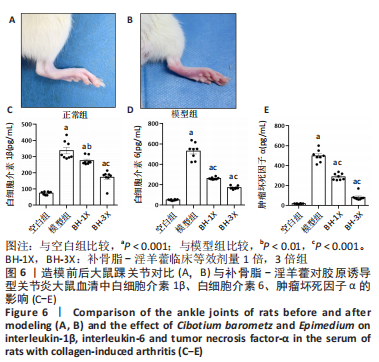
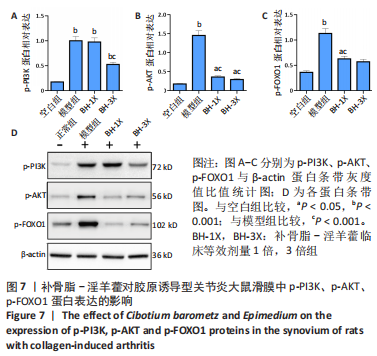
[13] 苏清君,李鹏,边朝辉,等.补骨脂素通过炎症和Ras/Raf1介导对类风湿关节炎大鼠的保护作用[J].世界中医药,2021,16(21):3180-3184+3190.
[14] 吴志明. 淫羊藿苷通过调控miR-223-3p/NLRP3信号轴缓解类风湿性关节炎的发生[D].南昌:南昌大学,2021.
[15] 吴志明,向艳茹,祝小波,等.淫羊藿苷治疗类风湿关节炎作用机制的研究进展[J].风湿病与关节炎,2022,11(7):71-74+80.
[16] WONG BR, JOSIEN R, LEE SY, et al. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med. 1997;186(12):2075-2080.
[17] 鲁亚奇,张晓,王金金,等.补骨脂化学成分及药理作用研究进展[J].中国实验方剂学杂志,2019,25(3):180-189.
[18] 曾华婷,郭健,陈彦.淫羊藿素药理作用及其新型给药系统的研究进展[J].中草药,2020,51(20):5372-5380.
[19] 肖涟波. 自拟蠲痹补肾方对类风湿关节炎炎症及骨破坏的防治作用[Z]. 上海: 上海中医药大学附属光华医院,2016-02-01.
[20] CONFORTI A, DI COLA I, PAVLYCH V, et al. Beyond the joints, the extra-articular manifestations in rheumatoid arthritis. Autoimmun Rev. 2021;20(2):102735.
[21] CATRINA AI, YTTERBERG AJ, REYNISDOTTIR G, et al. Lungs, joints and immunity against citrullinated proteins in rheumatoid arthritis. Nat Rev Rheumatol. 2014; 10(11):645-653.
[22] WELLS PM, WILLIAMS FMK, MATEY H, et al. RA and the microbiome: Do host genetic factors provide the link? J Autoimmun. 2019;99:104-115.
[23] 宋在鑫,柳清泳,王晓军,等.补肾通络汤联合甲氨蝶呤对类风湿关节炎患者炎症细胞因子、骨代谢标志物及血管新生相关因子的影响[J].现代生物医学进展,2020,20(19):3774-3778+3783.
[24] 苏清君,李鹏,边朝辉,等.补骨脂素通过炎症和Ras/Raf1介导对类风湿关节炎大鼠的保护作用[J].世界中医药,2021,16(21):3180-3184+3190.
[25] 王义翠,彭慧霞,夏子岚,等.淫羊藿苷药理作用及应用研究进展[J].中华中医药学刊:1-13[2023-02-04].
[26] 邱国伟,肖涟波,施杞.蠲痹强骨方对类风湿关节炎炎症和骨破坏的作用及机制研究进展[J].中医药临床杂志,2021,33(5):992-999.
[27] 殷方明,肖涟波,张昀,等.柚皮苷抑制胶原诱导小鼠关节炎症作用机制的实验研究[J].中国骨与关节损伤杂志,2016,31(3):285-288.
[28] 王宇,蒋嘉明,孔思远,等.补骨脂ADME及其相关毒性的研究进展[J].世界科学技术-中医药现代化,2017,19(2):276-281.
[29] BAIER A, MEINECKEL I, GAY S, et al. Apoptosis in rheumatoid arthritis. Curr Opin Rheumatol. 2003;15:274-279.
[30] YAMANISHI Y, BOYLE DL, GREEN DR, et al. p53 tumor suppressor gene mutations in fibroblast-like synoviocytes from erosion synovium and non-erosion synovium in rheumatoid arthritis. Arthritis Res Ther. 2004;7:R12-R18.
[31] 李生贵,潘正启,叶铄,等.circGFRA1靶向miR-642a-5p调控类风湿关节炎滑膜成纤维细胞增殖、迁移和侵袭的机制研究[J].中国细胞生物学学报,2022, 44(7):1276-1284.
[32] 康静婷,纪超.加兰他敏调节IL-1β/IL-1RA比率改善炎症微环境的机制探讨[J].J Chin Pharm Sci. 2022,31(10):773-781.
[33] 高晓珺,解骏,肖涟波,等.TNF-α拮抗剂对胶原诱导大鼠类风湿关节炎骨破坏治疗作用的实验研究[J].中国骨质疏松杂志,2013,19(5):458-464.
[34] 贺龙刚,高奥,林晓辉,等.青藤碱联合TNF-α抑制剂对RA模型大鼠骨破坏及体外破骨细胞活化的的影响[J].时珍国医国药,2021,32(10):2349-2352.
[35] 徐琼,张恒,王辉.基于EGFR介导的信号通路探讨中药活性成分抗肿瘤研究进展[J].中国实验方剂学杂志,2023,29(6):246-253.
[36] WANG Y, LYU Z, QIN Y, et al. FOXO1 promotes tumor progression by increased M2 macrophage infiltration in esophageal squamous cell carcinoma. Theranostics. 2020;10(25):11535-11548.
[37] 张小娜,杜小正,袁博,等.TRAF6泛素化调控功能与RA发病机制的研究进展[J].中国免疫学杂志:1-15[2022-12-03].
[38] PERLMAN H, GEORGANAS C, PAGLIARI LJ, et al. Bcl-2 expression in synovial fibroblasts is essential for maintaining mitochondrial homeostasis and cell viability. J Immunol. 2000;164(10):5227-5235.
[39] YU DH, LIU L, LU F, et al. Effect of total saponin of dioscoreae nipponicae rhizoma on PI3K/Akt signal pathway by in rIL-1β induced fibroblast-like synoviocytes. Chin J Exp Trad Med Formaul. 2012;18:199-202.
[40] PAN DM, WANG Q, CAI XD, et al. Duanteng Yimu Decoction induces apoptosis through inhibition of PI3K/Akt pathway in rheumatoid arthritis fibroblast-like synoviocytes. Chin J Trad Chin Med Pharm. 2018;33:2051-2055.
[41] ZOU LH, HOU CF, CHEN ZH, et al. Effects of Shuangwuxuanbi granule on the apoptosis of rheumatoid arthritis fibroblast-like synoviocytes and PI3K/Akt signaling pathway. Med J Chin PLA. 2018;43:721-726.
[42] LIU Y, JIANG SS, CHEN XY, et al. Effect of Hei Gu Teng Zhui Feng Huo Luo Capsule on PI3K/AKT/HIF-1α protein signaling pathway in rheumatoid arthritis rats. Chin J Immun. 2019;35:2206-2212.
[43] 王曼姝,孙思彤,袁宇,等.功能代谢组学在中药毒性评价中的研究策略及其应用[J].中草药,2023,54(2):349-358.
[44] 唐进法,牛璐,王晓艳,等.补骨脂醇提物中8种成分在正常和脂多糖模型大鼠体内的组织分布比较研究[J].中国中药杂志,2022,47(24):6763-6779.
[45] 时潇丽,杨德超,徐光临,等.含淫羊藿、补骨脂中成药的用药特点分析[J].中成药,2022,44(8):2744-2749.
[46] 颜冬梅,陈家辉,卢文滢,等.基于2020年版《中国药典》的含补骨脂成方制剂分析[J].亚太传统医药,2022,18(11):33-38. |