中国组织工程研究 ›› 2023, Vol. 27 ›› Issue (6): 928-934.doi: 10.12307/2023.309
• 干细胞综述 stem cell review • 上一篇 下一篇
外胚层间充质干细胞的获取及应用
张岐剑,徐希明
- 江苏大学药学院,江苏省镇江市 212000
-
收稿日期:2022-04-09接受日期:2022-06-08出版日期:2023-02-28发布日期:2022-08-12 -
通讯作者:徐希明,男,博士,教授,江苏大学药学院,江苏省镇江市 212000 -
作者简介:张岐剑,男,1996年生,江西省赣州市人,汉族,江苏大学药学院在读硕士,主要从事基因与组织工程研究。 -
基金资助:国家自然科学基金项目(81720108030),项目负责人:徐希明
Acquisition and application of ectodermal mesenchymal stem cells
Zhang Qijian, Xu Ximing
- School of Pharmacy, Jiangsu University, Zhenjiang 212000, Jiangsu Province, China
-
Received:2022-04-09Accepted:2022-06-08Online:2023-02-28Published:2022-08-12 -
Contact:Xu Ximing, MD, Professor, School of Pharmacy, Jiangsu University, Zhenjiang 212000, Jiangsu Province, China -
About author:Zhang Qijian, Master candidate, School of Pharmacy, Jiangsu University, Zhenjiang 212000, Jiangsu Province, China -
Supported by:the National Natural Science Foundation of China, No. 81720108030 (to XXM)
摘要:
文题释义:
间充质干细胞:是干细胞的一种,具有亚全能分化潜能,在特定的体内外环境下能够诱导分化成为多种间质组织细胞。间充质干细胞具有干细胞的共性,即自我更新、多向分化和归巢的能力。
外胚层:外胚层系来源于卵细胞动物极附近的细胞浆,原肠胚形成时开始出现在胚的外表面或表面,最终分化成表皮与神经系统。
背景:外胚层间充质干细胞是一种成体干细胞,获取便捷、来源丰富,在机体组织修复与免疫调节等方面发挥着重要作用。
目的:总结综述外胚层间充质干细胞获取与应用方面的最新进展。
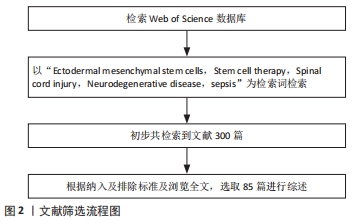
方法:检索Web of Science数据库中相关文章,检索词为“Ectodermal mesenchymal stem cells,Stem cell therapy,Spinal cord injury,Neurodegenerative disease,sepsis”,检索要求为研究原著与综述,检索时限为数据库建库至2021年,筛选出相关文献85篇,结合文献追溯法对所得文献进行分析总结。
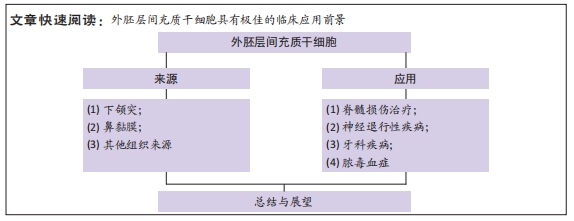
结果与结论:①外胚层间充质干细胞来源丰富、获取简便,其来源可以是牙组织、鼻黏膜和角膜组织等。与中胚层间充质干细胞相同的是,它们都具备多系分化能力和免疫调节功能。②而外胚层间充质干细胞与牙组织与神经组织具有同源性,因此具备更好的牙细胞、神经细胞分化能力,在牙组织修复、脊髓损伤修复、神经退行性疾病治疗等方面具有独特的优势,因此具备极为广阔的应用前景。③外胚层间充质干细胞的免疫调节功能同样不能忽视,目前虽仅有少量文献报道将其应用于脓毒血症等免疫疾病,但研究发现其免疫调节机制与中胚层间充质干细胞等并无二致,同时其还具备获取方式相对低损伤性等优势,因此外胚层间充质干细胞具有极佳的临床应用前景。
https://orcid.org/0000-0001-8076-7794 (张岐剑);https://orcid.org/0000-0002-7113-6226 (徐希明)
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中图分类号:
引用本文
张岐剑, 徐希明. 外胚层间充质干细胞的获取及应用[J]. 中国组织工程研究, 2023, 27(6): 928-934.
Zhang Qijian, Xu Ximing. Acquisition and application of ectodermal mesenchymal stem cells[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 928-934.
在外胚层的干细胞中,有一种易分离、易培养、增殖迅速且具有三系分化能力的细胞系,即外胚层间充质干细胞(ectodermal mesenchymal stem cells,EMSCs)。外胚层间充质干细胞不但符合间充质干细胞的标准,且来源更加丰富,获取更加便捷,例如口腔中牙髓、腭黏膜,鼻腔中鼻中隔黏膜和嗅球部黏膜等[15-18],都已有研究发现其中具有广泛的间充质干细胞。
2.2 外胚层间充质干细胞的获取
2.2.1 下颌突间充质干细胞 颌突是外胚层发育而来的面部结构,其中蕴含多种能够自我更新的干细胞,这些干细胞中存在最为广泛且丰富的便是间充质干细胞。来自晚期胚胎下颌突的间充质干细胞是最早报道的外胚层间充质干细胞之一,例如早期文献报道了一种外胚层间充质干细胞的分离方法,将BALB/c57小鼠胎鼠的下颌突中分离出组织块,胰酶消化出游离细胞,利用间充质干细胞的易贴壁性去除掉上皮细胞后,培养一段时间后细胞迅速扩增,表达了间充质干细胞标记物,且具有三系分化能力,是一种典型外胚层间充质干细胞[19-20]。
牙组织是下颌突发育而来的一种重要组织,其中同样蕴含丰富的间充质干细胞,例如已有研究报道从龋齿患者的牙齿中也成功分离出间充质干细胞,说明即使成年后,由下颌突发育来的组织中也存在大量间充质干细胞[21]。除牙齿外,牙周韧带里也有研究成功分离出了间充质干细胞[22]。近年来的研究显示,下颌突来源的间充质干细胞增殖速度要远高于中胚层间充质干细胞,虽然成骨性低于后者,但结合其获取来源的低损伤性,下颌突间充质干细胞依旧有着不错的临床应用前景[23]。
2.2.2 鼻黏膜间充质干细胞 鼻中隔也是颅神经嵴分化而来的其中一种的组织,附着其上的鼻黏膜具有很强的再生能力,间充质干细胞在鼻黏膜再生中发挥着重要作用。又因为鼻黏膜的可再生性,所以自人体获取该组织并分离培养间充质干细胞的损伤性较低,因此鼻黏膜间充质干细胞的临床应用前景要优于其他间充质干细胞。早期研究普遍是从胚胎或胎鼠鼻部结构中分离外胚层充质干细胞,例如有研究在晚期胚胎面部突起处成功定位了一种间充质干细胞,高表达神经嵴标记物p75及诸多间充质干细胞标记物,但无法确定该细胞的最终归属[24]。不久后便有研究成功地从幼年大鼠的鼻黏膜上分离出一种间充质干细胞,该细胞高表达神经嵴细胞标记物和间充质干细胞标记物,包括SOX1,Sall4,snail,sox10,S100β,CD133,nestin,vimentin,CD44和整合素β1等,是一种典型的外胚层间充质干细胞[25]。鼻腔组织中的间充质干细胞自此开始逐渐受到重视,自其他鼻腔黏膜组织中分离出间充质干细胞的报道也在增多,例如ALIZADEH等[26]从人鼻嗅球部黏膜组织中分离出了一种间充质干细胞,同样表达诸多间充质干细胞标记物,使用音猬因子SHH、成纤维生长因子8和碱性成纤维细胞生长因子等因子混合诱导可以使其向神经元样细胞分化。
2.2.3 其他外胚层组织来源间充质干细胞 除上述2种组织来源的间充质干细胞外,许多其他的外胚层组织中也发现了间充质干细胞,如有研究发现眼球角膜角质中存在着许多间充质干细胞,表达CD90,CD73和CD105等间充质干细胞标记蛋白,对角膜组织修复具有促进作用[27-29]。皮肤及附属器官也是一种外胚层组织,其中也含有一定量的外胚层间充质干细胞[30],但是由于真皮层存在大量的成纤维细胞的干扰,从中分离间充质干细胞费时费力,因而对其研究较少[31]。
2.3 外胚层间充质干细胞的应用 干细胞治疗研究由来已久,自20世纪60年代加拿大科学家麦卡洛克和蒂尔发现以来,干细胞一直都是研究热点,在再生医学领域发挥着不可或缺的作用。干细胞具有多向分化潜能,增殖迅速,是一类理想的种子细胞来源,广泛应用于组织工程领域。然而,干细胞移植受免疫微环境影响,易受免疫清除,同时炎性环境易导致干细胞过早凋亡而丧失治疗作用,因此,如何使机体免疫微环境对移植干细胞“兼容”已成为干细胞治疗的一大难题。
间充质干细胞除具有其他干细胞特性,如自我更新及多向分化外,还具备一定的免疫调节功能,对免疫相关疾病治疗起到一定的辅助作用[32-33]。过去很多研究认为间充质干细胞具有低免疫原性,所以进行异体间充质干细胞移植时不会引发强烈的免疫排斥反应[34]。近年来也有研究认为间充质干细胞的免疫逃避作用也是其具有高组织相容性的原因[35],研究发现间充质干细胞可以调节周边白细胞介素2水平,减少T细胞增殖从而下调免疫反应[36],因此,间充质干细胞还可以作为器官移植的辅助细胞,降低移植后的排斥反应。
另外在机体受损伤刺激后,间充质干细胞借助外泌体释放细胞因子改变了受损部位微环境,可以诱导自体细胞对损伤进行修复[37-38],而这一切功能的实现都是基于间充质干细胞与环境的相互作用。因此,研究者们可把间充质干细胞当作一种生物智能材料,它在损伤过程中填补细胞缺失状态,其表现的生物活性不仅体现在环境刺激导致的细胞性状改变,也同时不断向外释放生物大分子,改善损伤部位细胞生长微环境。与传统高分子材料的缓释作用不同,间充质干细胞释放生物大分子的速率随损伤进程动态变化,对损伤部位微环境具有“智能”调节作用,最终将重构外界刺激造成的失稳状态,达到一种动态平衡,同时活细胞对刺激反应的速度与强度都要优于传统高分子材料[39]。
尽管间充质干细胞的应用范围广泛,但是作为一种初步分化的成体干细胞,其跨胚层治疗效果有限,例如中胚层间充质干细胞只能延缓脊髓损伤病程进展,其治疗效果不如外胚层间充质干细胞,两者虽都能够减少损伤处的星形胶质瘢痕产生,但近期研究发现后者在体内外转分化为神经细胞的能力都要更强[40-41],能够有效促进损伤处的神经连接,从而改善继发损伤阶段的脊髓神经功能。外胚层间充质干细胞来源丰富,其来源可以是末端神经感受器及其附属器官,相较于中胚层间充质干细胞获取更加简便,不会造成严重的手术创伤,提高了外胚层间充质干细胞临床应用的可行性。同时研究发现外胚层间充质干细胞更易于诱导分化为其他外胚层细胞[42],因此该细胞在特定疾病的细胞治疗中有着独特的优势,具有极高的研究价值。
2.3.1 治疗脊髓损伤 脊髓是中枢神经系统的重要组成部分,其结构与组成十分复杂。外源性损伤对脊髓造成的直接损害是神经细胞的大量丢失,所以寻找具有神经修复功能的种子细胞是组织工程治疗脊髓损伤的核心所在,同时脊髓损伤发生时抑制性微环境以及星形胶质瘢痕的产生[43-44],也是阻碍脊髓损伤恢复的关键因素。
目前为止,已有许多研究报道了外胚层间充质干细胞诱导成为神经细胞的案例,这直接有益于脊髓损伤的治疗,例如张志坚团队在鼻黏膜间充质干细胞基础上使用腺病毒构建了谷胺酰胺酶2-鼻黏膜间充质干细胞表达体系,使得间充质干细胞能够表达转谷胺酰胺酶2,提高细胞增殖能力的同时,谷胺酰胺酶2形成的自交联体系的稳固与缓释作用,防止了生长因子过早流失,促进了外胚层间充质干细胞向神经的分化[45]。随着3D打印技术的兴起,3D仿生打印脊髓治疗脊髓损伤已逐渐形成一种趋势,因其可以精准构造神经再生微环境,可以改善脊髓损伤处新生轴突生长紊乱的状态,所以对脊髓损伤的修复效果要好于常规支架[46-47],同时得益于外胚层间充质干细胞的神经分化能力,近年来许多结合外胚层间充质干细胞与3D打印支架治疗脊髓损伤的策略,都取得了不错的效果[48]。目前2种外胚层间充质干细胞治疗脊髓损伤的策略,见图3。
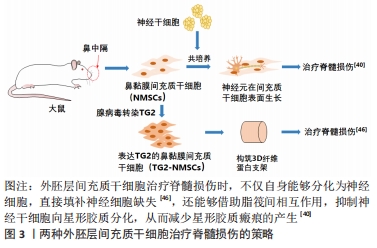
星形胶质细胞增生及小胶质细胞的过度M1极化是脊髓损伤治疗的2大难点,有研究发现,鼻黏膜来源的间充质干细胞与原代神经干细胞共培养,通过脂筏部位的相互作用,可有效抑制神经干细胞向星型胶质细胞分化,并促进其向神经元分 化[39]。YU等[49]同样以此为种子细胞,使用枸杞寡糖和外胚层间充质干细胞构建可注射纤维蛋白原凝胶复合支架(LBO-EMSCs-Fibrin),发现枸杞寡糖可促进外胚层间充质干细胞的旁分泌作用,并通过上调PI3K-Akt-mTOR通路促进M1型小胶质细胞向M2型转化。
近年来有研究发现,外胚层间充质干细胞分泌的胞外囊泡可以使体外培养的星形胶质细胞向神经元样细胞转化,同时提高其神经元标记物神经丝蛋白和神经元特异性烯醇化酶的表达[50],诱导损伤处激活的星形胶质细胞转分化为神经元也给脊髓损伤乃至更多中枢神经系统疾病的治疗提供了新思路。
2.3.2 治疗神经退行性疾病 神经退行性疾病是多种诱因引发的神经元和(或)髓鞘缺失,其表现为部分神经功能丧失,严重情况下可能导致死亡,长期损伤可能导致阿尔茨海默症、帕金森症和肌萎缩性侧索硬化等临床无有效治疗手段的疾病。外胚层间充质干细胞有着一定的神经分化能力,因此有许多研究尝试使用外胚层间充质干细胞治疗神经退行性疾病,例如法国学者NIVET等[51]将人源嗅黏膜中分离的一种间充质干细胞,移植到脑损伤小鼠后,发现该细胞广泛分布于海马区且高表达成熟神经元标记微管相关蛋白2,表明该细胞可在受损海马区富集并向神经元分化,从而替代受损神经元,恢复小鼠记忆。
除外胚层间充质干细胞直接分化为神经元修复脑损伤外,有实验证明间充质干细胞的外泌体可以提高神经细胞中突触可塑性相关基因的表达,抑制神经炎症发生,刺激损伤区的神经再生,对神经退行性病变的扩散具有一定的抑制作用[52-54]。因此在脑病变处原位移植外胚层间充质干细胞,一方面通过细胞的神经分化填补神经细胞的缺损,另一方面借助这些细胞的外泌体同时抑制病程的发展有望成为神经退行性疾病治疗的新方案。
2.3.3 治疗牙科疾病 传统牙组织缺损治疗多采用拔牙后移植无机材料假牙的方式,如氧化锆和钛合金等低致敏性金属在临床上使用已十分广泛,虽然假牙的移植可以迅速恢复牙齿的部分功能,但是这种方式对牙周组织的损伤没有治疗作用,所以组织工程方法治疗牙组织缺损疾病仍有很高的研究价值。
近来,有研究发现骨髓间充质干细胞和脂肪间充质干细胞等中胚层间充质干细胞具有不错的成骨化能力,可作为骨组织修复的种子细胞,但是应用于牙组织修复却效果不佳,其原因可能是骨组织与牙组织来源于不同胚层,二者骨质组成有着本质区别。
牙组织是由外胚层发育而来,因此,外胚层间充质干细胞相较于其他来源间充质干细胞理应具有显著优势。目前,已有研究表明该细胞在牙组织修复中具有良好的治疗效果[55]。外胚层间充质干细胞中高表达成骨相关基因——Runt相关转录因子2/核心结合因子α1(Runx2/Cbfa1),osterix(Osx)以及p75神经因子受体(p75NTR)[56-57],这或许是外胚层间充质干细胞治疗牙组织疾病的理论依据。
2.3.4 治疗脓毒症 脓毒症是一种由病原微生物及毒素感染而引发的全身炎症反应综合征。国际重症医学会将临床上脓毒症分为全身炎性反应综合征、脓毒症、严重脓毒症及脓毒症休克4个阶段,发展过程涉及多个脏器的免疫损伤,后期发展为多器官功能障碍综合征 [58]。据统计,全球脓毒症患者每年约为1 900万,且以1.5%的速率逐年递增,而死亡率则高达29.3%-30.9%[59-60]。
脓毒症病程特点:主要分为2个阶段,即过度炎症反应期和免疫麻痹期[61]。过度炎症反应期主要是由于病原体进入宿主体内导致的免疫稳态失调。在此期间,模式识别受体被病原相关分子模式激活,进而引发多种炎症因子的表达,主要包括:肿瘤坏死因子α、白细胞介素1β、白细胞介素2、白细胞介素6、白细胞介素8和干扰素γ等,而这些因子的过度表达则会导致“炎症风暴”的产生[62]。过去数十年对于脓毒症治疗的研究重点主要为抑制早期的过度炎症反应,然而,这些研究仅能降低脓毒症早期的死亡率。由于炎症因子过度释放,固有免疫细胞发生大量焦亡,致使部分患者进入了免疫抑制阶段,对于病原体的二次感染缺乏抵抗能力,即免疫麻痹期[63],而该阶段的死亡率高达70%,表现出病理复杂、病程凶险及预后不佳等特点。间充质干细胞除自身特有的多向分化能力以外,其旁分泌作用也逐渐为人们所重视。间充质干细胞可通过旁分泌作用分泌大量细胞营养因子及免疫调节因子,通过对免疫微环境的调节治疗脓毒症。而间充质干细胞的免疫调节作用主要体现在对巨噬细胞和T细胞的驯化上。
间充质干细胞调节巨噬细胞极化作用:巨噬细胞是一类具有异质性的免疫细胞。未活化的巨噬细胞称为M0型巨噬细胞,负责免疫监视;而活化后的巨噬细胞根据激活后的状态和功能不同,分为经典激活巨噬细胞(M1型)和替代激活巨噬细胞(M2型)[64]。M1型巨噬细胞上调免疫活性,促进炎症反应,发挥防御功能;M2型巨噬细胞抑制免疫活性,降低炎症反应,发挥组织损伤修复功能[65-66]。有大量肿瘤相关研究表明巨噬细胞可被驯化为M2型,通过分泌免疫抑制因子促进肿瘤的生长和转移[67-68]。KIM等[69]首先提出了“间充质干细胞驯化巨噬细胞”这一概念,已有研究尝试通过骨髓来源间充质干细胞与巨噬细胞共培养,提高M2型巨噬细胞比例,使得白细胞介素10水平升高。另有研究表明,在小鼠体内输入间充质干细胞驯化的巨噬细胞可以减少在结肠炎中小鼠的死亡和体质量减少,提高脓毒症小鼠的生存率[70]。此外,间充质干细胞条件培养基也可促进M2巨噬细胞的增殖从而减轻由急性呼吸综合征、脓毒症以及新冠病毒引起的肺损伤[71]。除了大量M1巨噬细胞浸润,巨噬细胞大量焦亡也是引起免疫麻痹的重要原因之一。有研究表明,多种病原相关分子模式可促进炎症复合体3的组装[72],而后者可剪切并激活caspase-1,从而介导经典焦亡途径[73]。因此,间充质干细胞对于脓毒症早期的过度炎症反应起到了良好的控制作用。
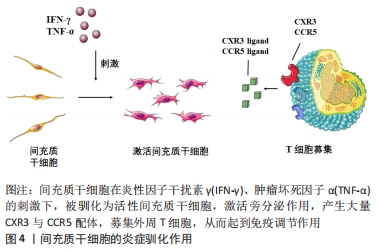
间充质干细胞调节T细胞分化的作用:T细胞是免疫系统中重要的一类效应细胞,在局部免疫微环境的调节方面具有重要作用。间充质干细胞对于T细胞分化的调节方式有两种:①间接调节,即通过自身的旁分泌作用使一些其他固有免疫细胞,如巨噬细胞,树状细胞,单核细胞维持在未成熟期从而抑制效应T细胞(effective T cells,Teff)的激活,并促进调节T细胞(regulatory T cell,Treg)的形成[74];②直接调节,即通过分泌炎症抑制因子,如一氧化氮、转化生长因子β、血红素氧化酶1和白血病抑制因子等[75-78],抑制毒性CD8+T细胞的激活、辅助型T细胞1(helper T cell 1,Th1),辅助型T细胞17(Th17)的分化,并促进CD4+CD25+FOXP3+ T细胞及白细胞介素10+Treg的分化[79-80]。有研究表明,炎性驯化作用通过激活iNOS-IDO轴,可显著增强间充质干细胞的旁分泌效应并产生大量趋化因子如CCL5,CXCL-9,CXCL-10和CXCL-11,募集外周T细胞,并分泌更多的抗炎介质进一步促进Treg的形成并减少Teff,Th1和Th17的数量,促进组织再生[78],见图4。
目前已有研究表明,牙髓来源间充质干细胞可有效促进Treg的形成,通过创造免疫抑制微环境来缓解脓毒症造成的多器官衰竭[81]。值得注意的是,间充质干细胞对于T细胞的调控与T细胞所处的状态有关,对于正常T细胞,间充质干细胞会抑制其增殖,而对于脂多糖作用后的T细胞,间充质干细胞可显著提高其存活率,并促进其增殖并分泌干扰素γ[82]。而干扰素γ是逆转免疫麻痹重要的因子之一。因此,间充质干细胞对于T细胞的具有高度调控作用,且具有选择性。
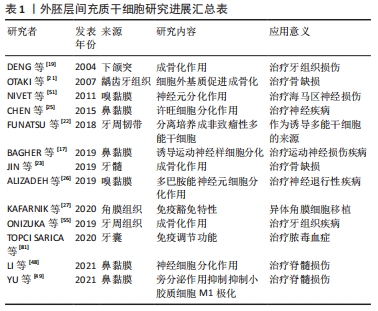
作者总结了外胚层间充质干细胞的研究进展,见表1。
| [1] FRIEDENSTEIN AJ, CHAILAKHJAN RK, LALYKINA KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3(4):393-403. [2] ROBINSON D, EFRAT M, MENDES D, et al. Implants composed of carbon fiber mesh and bone-marrow-derived, chondrocyte-enriched cultures for joint surface reconstruction. Bull Hosp Jt Dis Orthop Inst. 1993;53(1):75-82. [3] JIANG Y, JAHAGIRDAR B, REINHARDT R, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002; 418(6893):41-49. [4] ZUK P, ZHU M, ASHJIAN P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279-4295. [5] ERICES A, CONGET P, MINGUELL J. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109(1):235-242. [6] HORWITZ EM, BLANC KL, DOMINICI M, et al. Clarification of the nomenclature for MSC: the international society for cellular therapy position statement. Cytotherapy. 2005;7(5):393-395. [7] DOMINICI M, LE BLANC K, MUELLER I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. the international society for cellular therapy position statement. Cytotherapy. 2006; 8(4):315-317. [8] LIU Y, WANG H, DOU H, et al. Bone regeneration capacities of alveolar bone mesenchymal stem cells sheet in rabbit calvarial bone defect. J Tissue Eng. 2020;11:2041731420930379. [9] WU X, ZHANG S, LAI J, et al. Therapeutic potential of bama pig adipose-derived mesenchymal stem cells for the treatment of carbon tetrachloride-induced liver fibrosis. Exp Clin Transplant. 2020;18(7): 823-831. [10] NIU Y, WANG X, LI M, et al. Exosomes from human umbilical cord mesenchymal stem cells attenuates stress-induced hippocampal dysfunctions. Metab Brain Dis. 2020;35(8):1329-1340. [11] BONNET M, GUIRAUDIE-CAPRAZ G, MARQUESTE T, et al. Immediate or delayed transplantation of a vein conduit filled with nasal olfactory stem cells improves locomotion and axogenesis in rats after a peroneal nerve loss of substance. Int J Mol Sci. 2020;21(8):1-17. [12] BAGHER Z, EHTERAMI A, NASROLAHI M, et al. Hesperidin promotes peripheral nerve regeneration based on tissue engineering strategy using alginate/chitosan hydrogel: in vitro and in vivo study. Int J Polym Mater. 2021;70(5):299-308. [13] BETTERS E, CHARNEY RM, GARCIA-CASTRO MI. Early specification and development of rabbit neural crest cells. Dev Biol. 2018;444:S181-S192. [14] ZEUNER MT, DIDENKO NN, HUMPHRIES D, et al. Isolation and characterization of neural crest-derived stem cells from adult ovine palatal tissue. Front Cell Dev Biol. 2018;6:39. [15] SUI B, CHEN C, KOU X, et al. Pulp stem cell-mediated functional pulp regeneration. J Dent Res. 2019;98(1):27-35. [16] NAUNG NY, DUNCAN W, DE SILVA R, et al. Localization and characterization of human palatal periosteum stem cells in serum-free, xeno-free medium for clinical use. Eur J Oral Sci. 2019;127(2):99-111. [17] BAGHER Z, ATOUFI Z, ALIZADEH R, et al. Conductive hydrogel based on chitosan-aniline pentamer/gelatin/agarose significantly promoted motor neuron-like cells differentiation of human olfactory ecto-mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl. 2019;101: 243-253. [18] MAZETO ERCOLIN AC, SANTOS ROBALLO KC, CASALS JB, et al. Rabbit olfactory stem cells. isolation protocol and characterization. Acta Cir Bras. 2016;31(1):59-66. [19] DENG M, JIN Y, SHI J, et al. Multilineage differentiation of ectomesenchymal cells isolated from the first branchial arch. Tissue Eng. 2004;10(9):1597-1606. [20] YAN Z, LIN Y, JIAO X, et al. Characterization of ectomesenchymal cells isolated from the first branchial arch during multilineage differentiation. Cells Tissues Organs. 2006;183(3):123-132. [21] OTAKI S, UESHIMA S, SHIRAISHI K, et al. Mesenchymal progenitor cells in adult human dental pulp and their ability to form bone when transplanted into immunocompromised mice. Cell Biol Int. 2007; 31(10):1191-1197. [22] FUNATSU T, GOMI K, MATSUSHIMA Y, et al. Characterization of mesenchymal stem cells derived from periodontal ligament. J Hard Tissue Biol. 2018;27(2):131-137. [23] JIN Q, YUAN K, LIN W, et al. Comparative characterization of mesenchymal stem cells from human dental pulp and adipose tissue for bone regeneration potential. Artif Cells Nanomed Biotechnol. 2019; 47(1):1577-1584. [24] WEN X, LIU L, DENG M, et al. Characterization of p75(+) ectomesenchymal stem cells from rat embryonic facial process tissue. Biochem Biophys Res Commun. 2012;427(1):5-10. [25] CHEN Q, ZHANG Z, LIU J, et al. A fibrin matrix promotes the differentiation of emscs isolated from nasal respiratory mucosa to myelinating phenotypical schwann-like cells. Mol Cells. 2015;38(3): 221-228. [26] ALIZADEH R, ZARRINTAJ P, KAMRAVA S, et al. Conductive hydrogels based on agarose/alginate/chitosan for neural disorder therapy. Carbohydr Polym. 2019;224:115161. [27] KAFARNIK C,MCCLELLAN A,DZIASKO M, et al. Canine corneal stromal cells have multipotent mesenchymal stromal cell properties in vitro. Stem Cells Dev. 2020;29(7):425-439. [28] KHOROLSKAYA JI, PEREPLETCHIKOVA DA, KACHKIN DV, et al. Derivation and characterization of egfp-labeled rabbit limbal mesenchymal stem cells and their potential for research in regenerative ophthalmology. Biomedicines. 2021;9(9):1-17. [29] SHUKLA S, SHANBHAG SS, TAVAKKOLI F, et al. Limbal epithelial and mesenchymal stem cell therapy for corneal regeneration. Curr Eye Res. 2020;45(3):265-277. [30] TOMA J, AKHAVAN M, FERNANDES K, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3(9):778-784. [31] ICHIM TE, O’HEERON P, KESARI S. Fibroblasts as a practical alternative to mesenchymal stem cells. J Transl Med. 2018;16(212):1-9. [32] HARRELL CR, DJONOV V, VOLAREVIC V. The cross-talk between mesenchymal stem cells and immune cells in tissue repair and regeneration. Int J Mol Sci. 2021;22(5):1-13. [33] HAN Y, LI X, ZHANG Y, et al. Mesenchymal stem cells for regenerative medicine. Cells. 2019;8(886):1-32. [34] GRIFFIN MD, RITTER T, MAHON BP. Immunological aspects of allogeneic mesenchymal stem cell therapies. Hum Gene Ther. 2010;21(12):1641-1655. [35] ANKRUM J, ONG J, KARP J. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252-260. [36] FANG L, LANGE C, ENGEL M, et al. Sensitive balance of suppressing and activating effects of mesenchymal stem cells on t-cell proliferation. Transplantation. 2006;82(10):1370-1373. [37] KALLURI R, LEBLEU V. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):1-40. [38] ZHANG S, CHUAH S, LAI R, et al. Msc exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16-27. [39] DENG W, SHAO F, HE Q, et al. Emscs build an all-in-one niche via cell-cell lipid raft assembly for promoted neuronal but suppressed astroglial differentiation of neural stem cells. Adv Mater. 2019;31(10):1-10. [40] DILGER N, NEEHUS A, GRIEGER K, et al. Gap junction dependent cell communication is modulated during transdifferentiation of mesenchymal stem/stromal cells towards neuron-like cells. Front Cell Dev Biol. 2020;8:869. [41] AFIZADEH R, BAGHER Z, KAMRAVA SK, et al. Differentiation of human mesenchymal stem cells (msc) to dopaminergic neurons: a comparison between wharton’s jelly and olfactory mucosa as sources of mscs. J Chem Neuroanat. 2019;96:126-133. [42] PRIESTER C, MACDONALD A, DHAR M, et al. Examining the characteristics and applications of mesenchymal, induced pluripotent, and embryonic stem cells for tissue engineering approaches across the germ layers. Pharmaceuticals (Basel). 2020;13(11):1-27. [43] LI Y, HE X, KAWAGUCHI R, et al. Microglia-organized scar-free spinal cord repair in neonatal mice. Nature. 2020;587(7835):613-618. [44] YUAN Y, HE C. The glial scar in spinal cord injury and repair. Neurosci Bull. 2013;29(4):421-435. [45] SHI W, QUE Y, LV D, et al. Overexpression of tg2 enhances the differentiation of ectomesenchymal stem cells into neuron-like cells and promotes functional recovery in adult rats following spinal cord injury. Mol Med Rep. 2019;20(3):2763-2773. [46] KOFFLER J, ZHU W, QU X, et al. Biomimetic 3d-printed scaffolds for spinal cord injury repair. Nat Med. 2019;25(2):263-269. [47] BEDIR T, ULAG S, USTUNDAG CB, et al. 3d bioprinting applications in neural tissue engineering for spinal cord injury repair. Mater Sci Eng C Mater Biol Appl. 2020;110:110741. [48] LI Y, CAO X, DENG W, et al. 3d printable sodium alginate-matrigel (sa-ma) hydrogel facilitated ectomesenchymal stem cells (emscs) neuron differentiation. J Biomater Appl. 2021;35(6):709-719. [49] YU Q, LIAO M, SUN C, et al. Lbo-emsc hydrogel serves a dual function in spinal cord injury restoration via the pi3k-akt-mtor pathway. ACS Appl Mater Interfaces. 2021;13(41):48365-48377. [50] 贯世豪,黄永辉,龚爱华,等.外胚层间充质干细胞来源细胞外囊泡诱导大鼠星形胶质细胞向神经元的转分化[J].中国组织工程研究,2022,26(30):4840-4846. [51] NIVET E, VIGNES M, GIRARD SD, et al. Engraftment of human nasal olfactory stem cells restores neuroplasticity in mice with hippocampal lesions. J Clin Invest. 2011;121(7):2808-2820. [52] CHEN Y, LU C, KE C, et al. Mesenchymal stem cell-derived exosomes ameliorate alzheimer’s disease pathology and improve cognitive deficits. Biomedicines. 2021;9(6):1-19. [53] REZA-ZALDIVAR EE, HERNANDEZ-SAPIENS MA, GUTIERREZ-MERCADO YK, et al. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of alzheimer’s disease. Neural Regen Res. 2019;14(9):1626-1634. [54] LI Q, WANG Z, XING H, et al. Exosomes derived from mir-188-3p-modified adipose-derived mesenchymal stem cells protect parkinson’s disease. Mol Ther Nucleic Acids. 2021;23:1334-1344. [55] ONIZUKA S, IWATA T. Application of periodontal ligament-derived multipotent mesenchymal stromal cell sheets for periodontal regeneration. Int J Mol Sci. 2019;20(11):1-13. [56] WANG W, YUAN C, LIU Z, et al. Characteristic comparison between canine and human dental mesenchymal stem cells for periodontal regeneration research in preclinical animal studies. Tissue Cell. 2020; 67:101405. [57] XING Y, NIE X, CHEN G, et al. Comparison of p75ntr-positive and -negative etcomesenchymal stem cell odontogenic differentiation through epithelial-mesenchymal interaction. Cell Prolif. 2016;49(2):185-194. [58] SINGER M, DEUTSCHMAN CS, SEYMOUR CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. [59] FUJISHIMA S, GANDO S, SAITOH D, et al. A multicenter, prospective evaluation of quality of care and mortality in japan based on the surviving sepsis campaign guidelines. J Infect Chemother. 2014;20(1): 115-120. [60] CZUPRYNA P, GARKOWSKI A, MONIUSZKO A, et al. Patients with sepsis in infectious diseases department in years 1997-2010 - epidemiology and clinical features. Przegl Epidemiol. 2013;67:429-434,535. [61] HOLLENBERG SM, SINGER M. Pathophysiology of sepsis-induced cardiomyopathy. Nat Rev Cardiol. 2021;18(6):424-434. [62] KUMAR V. Toll-like receptors in sepsis-associated cytokine storm and their endogenous negative regulators as future immunomodulatory targets. Int Immunopharmacol. 2020;89(Pt B):107087. [63] ANNANE D, BELLISSANT E, BOLLAERT PE, et al. Corticosteroids for severe sepsis and septic shock: a systematic review and meta-analysis. BMJ (Clinical research ed.). 2004;329(7464):480-480. [64] WANG N, LIANG H, ZEN K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol. 2014;5:614. [65] MUELLER CK, SCHULTZE-MOSGAU S. Histomorphometric analysis of the phenotypical differentiation of recruited macrophages following subcutaneous implantation of an allogenous acellular dermal matrix. Int J Oral Maxillofac Surg. 2011;40(4):401-407. [66] FUJIU K, MANABE I, NAGAI R. Renal collecting duct epithelial cells regulate inflammation in tubulointerstitial damage in mice. J Clin Invest. 2011;121(9):3425-3441. [67] RODRIGUEZ E, BOELAARS K, BROWN K, et al. Sialic acids in pancreatic cancer cells drive tumour-associated macrophage differentiation via the siglec receptors siglec-7 and siglec-9. Nat Commun. 2021;12(1):1270. [68] MALLER O, DRAIN AP, BARRETT AS, et al. Tumour-associated macrophages drive stromal cell-dependent collagen crosslinking and stiffening to promote breast cancer aggression. Nat Mater. 2021;20(4): 548-559. [69] KIM J, HEMATTI P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37(12):1445-1453. [70] ANDERSON P, SOUZA-MOREIRA L, MORELL M, et al. Adipose-derived mesenchymal stromal cells induce immunomodulatory macrophages which protect from experimental colitis and sepsis. Gut. 2013;62(8):1131-1141. [71] GORMAN E, MILLAR J, MCAULEY D, et al. Mesenchymal stromal cells for acute respiratory distress syndrome (ards), sepsis, and covid-19 infection: optimizing the therapeutic potential. Expert Rev Respir Med. 2021;15(3):301-324. [72] TROVA S, FENTON M, CHAUHAN B, et al. Human and pathogen derived ndpks act as novel damps and pamps to drive leukemia cell survival and progression through signaling via the tlr4-mediated alternative nlrp3 inflammasome pathway. Blood. 2019;134:2684. [73] NAJI A, MUZEMBO BA, YAGYU K, et al. Endocytosis of indium-tin-oxide nanoparticles by macrophages provokes pyroptosis requiring nlrp3-asc-caspase1 axis that can be prevented by mesenchymal stem cells. Sci Rep. 2016;6:26162. [74] MA S, XIE N, LI W, et al. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014;21(2):216-225. [75] SU J, CHEN X, HUANG Y, et al. Phylogenetic distinction of inos and ido function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death Differ. 2014;21(3):388-396. [76] CHABANNES D, HILL M, MERIEAU E, et al. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110(10):3691-3694. [77] AUGELLO A, TASSO R, NEGRINI S, et al. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35(5):1482-1490. [78] CAO W, YANG Y, WANG Z, et al. Leukemia inhibitory factor inhibits t helper 17 cell differentiation and confers treatment effects of neural progenitor cell therapy in autoimmune disease. Immunity. 2011;35(2): 273-284. [79] HSU W, LIN C, CHIANG B, et al. Prostaglandin e2 potentiates mesenchymal stem cell–induced il-10+ ifn-γ+ cd4+ regulatory T cells to control transplant arteriosclerosis. J Immunol. 2013;190(5):2372-2380. [80] HU J, ZHANG L, WANG N, et al. Mesenchymal stem cells attenuate ischemic acute kidney injury by inducing regulatory t cells through splenocyte interactions. Kidney Int. 2013;84(3):521-531. [81] TOPCU SARICA L, ZIBANDEH N, GENÇ D, et al. Immunomodulatory and tissue-preserving effects of human dental follicle stem cells in a rat cecal ligation and perforation sepsis model. Arch Med Res. 2020; 51(5):397-405. [82] LE BUREL S, THEPENIER C, BOUTIN L, et al. Effect of mesenchymal stromal cells on t cells in a septic context: immunosuppression or immunostimulation? Stem Cells Dev. 2017;26(20):1477-1489. [83] TIAN J, ZHU Q, ZHANG Y, et al. Olfactory ecto-mesenchymal stem cell-derived exosomes ameliorate experimental colitis via modulating Th1/Th17 and Treg cell responses. Front Immunol. 2020;11:598322. [84] TAKAHASHI K, YAMANAKA S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663-676. [85] WANG L, ZHU M, GUO Q, et al. Comparing the reprogramming efficiency of mouse embryonic fibroblasts, mouse bone marrow mesenchymal stem cells and bone marrow mononuclear cells to ipscs. In Vitro Cell Dev Biol Anim. 2012;48(4):236-243. |
| [1] | 潘钟杰, 秦志鸿, 郑铁军, 丁晓飞, 廖世杰. 股骨头坏死发病机制中非编码RNA的靶标性[J]. 中国组织工程研究, 2023, 27(9): 1441-1447. |
| [2] | 蔡志浩, 谢召勇. 股骨颈前倾角测量评估:如何建立统一的方法和标准[J]. 中国组织工程研究, 2023, 27(9): 1448-1454. |
| [3] | 党 祎, 杜成砚, 姚红林, 袁能华, 曹 金, 熊 山, 张顶梅, 王 信. 激素型骨坏死与氧化应激[J]. 中国组织工程研究, 2023, 27(9): 1469-1476. |
| [4] | 杨九杰, 李 治, 王树杰, 田 野, 赵 伟. 神经电生理监测硬脊膜切开减压治疗急性脊髓损伤过程中脊髓的功能变化[J]. 中国组织工程研究, 2023, 27(8): 1232-1236. |
| [5] | 王 继, 张 敏, 杨中亚, 张 龙. 体力活动干预2型糖尿病肌少症的研究现状[J]. 中国组织工程研究, 2023, 27(8): 1272-1277. |
| [6] | 聂晨晨, 苏凯奇, 高 静, 凡勇福, 阮晓迪, 袁 洁, 段昭远, 冯晓东. 环状RNA调控脑缺血发病的作用与机制[J]. 中国组织工程研究, 2023, 27(8): 1286-1291. |
| [7] | 高 煜, 韩佳慧, 葛 新. 脊髓缺血再灌注损伤后的免疫炎性微环境[J]. 中国组织工程研究, 2023, 27(8): 1300-1305. |
| [8] | 徐星星, 文超举, 孟茂花, 王勤英, 陈镜桥, 董 强. 口腔种植中的碳纳米材料[J]. 中国组织工程研究, 2023, 27(7): 1062-1070. |
| [9] | 李 诚, 郑国爽, 蒯贤东, 于炜婷. 海藻酸盐支架修复关节软骨[J]. 中国组织工程研究, 2023, 27(7): 1080-1088. |
| [10] | 陈世崧, 刘晓红, 徐志云. 人工生物瓣膜的研究现状及展望[J]. 中国组织工程研究, 2023, 27(7): 1096-1102. |
| [11] | 芦 笛, 张 成, 段荣泉, 刘宗响. 磷酸钙陶瓷骨修复材料的骨诱导性能[J]. 中国组织工程研究, 2023, 27(7): 1103-1109. |
| [12] | 史业弘, 王 成, 陈世玖. 小口径人工血管的早期血栓形成与预防[J]. 中国组织工程研究, 2023, 27(7): 1110-1116. |
| [13] | 唐昊天, 廖荣东, 田 京. 压电材料修复骨缺损的应用及设计思路[J]. 中国组织工程研究, 2023, 27(7): 1117-1125. |
| [14] | 郝柳芳, 段红梅, 王子珏, 郝 飞, 郝 鹏, 赵 文, 高钰丹, 杨朝阳, 李晓光. 转基因小鼠脊髓损伤后室管膜细胞的时空动态变化[J]. 中国组织工程研究, 2023, 27(6): 883-889. |
| [15] | 李晓寅, 杨晓青, 陈淑莲, 李正超, 王梓琪, 宋 震, 朱达仁, 陈旭义. 胶原/丝素蛋白支架联合神经干细胞治疗创伤性脊髓损伤[J]. 中国组织工程研究, 2023, 27(6): 890-896. |
间充质干细胞是广泛存在于人体的一种多能干细胞。FRIEDENSTEIN等[1]在1970年从骨髓中分离出一种细胞,形态类似于成纤维细胞,却有着不同的增殖速率与贴壁性,且易诱导为成骨细胞,这些细胞就是中胚层间充质干细胞。此后对骨髓中间充质干细胞的研究逐渐增多且日益完善[2-3],科学家们也从更多实质组织中发现了多种间充质干细胞[4-5]。2005年,国际细胞治疗组织正式提出“间充质干细胞”这一名词[6-7],并给出定义:间充质干细胞是一种贴壁细胞且必须表达CD105、CD73和CD90等表面分子,而不表达CD45,CD34,CD14,CD11b,CD79α,CD19或HLA-DR等表面分子;同时间充质干细胞应该表现出三系分化能力,即向成骨细胞、脂肪细胞及软骨细胞分化的能力。
间充质干细胞广泛存在于人体的多种组织与器官中,在机体的修复以及免疫调控中起到关键作用。目前为止,已有超过1 400项临床试验使用间充质干细胞,其适用领域包括骨质疾病、糖尿病、心血管疾病、自身免疫病及神经系统疾病等(数据来源自www.clinicaltrials.gov)。目前研究较多的间充质干细胞主要来源于中胚层组织如骨髓、脂肪和脐带等[8-10],而对于外胚层组织间充质干细胞的研究少有涉及。值得一提的是,随着研究的深入,人们已经认识到外胚层间充质干细胞的诸多优点,其应用范围也日趋拓宽,使得其研究价值不可忽视,近年来对其的研究也在逐渐增多。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
1.1.1 检索人及检索时间 第一作者在2022年1月进行检索。
1.1.2 检索文献时限 1983年9月至2021年12月。
1.1.3 检索数据库 Web of Science数据库。
1.1.4 检索词 检索词为“Ectodermal mesenchymal stem cells,Stem cell therapy,Spinal cord injury,Neurodegenerative disease,Sepsis,mesenchymal stem cells”。
1.1.5 检索文献类型 研究原著和综述。
1.1.6 检索策略 Web of Science数据库检索策略,见图1。
1.2 入组标准
1.2.1 纳入标准 ①与外胚层间充质干细胞研究相关的文献;②近期发表的,实验方法和数据可信、结果与结论可靠的研究论文及综述。
1.2.2 排除标准 排除重复性研究、研究内容不相符及未正式发表的文献。
1.3 文献质量评估与数据提取 共检索到文献 300篇,排除与研究目的相关性差、内容重复及不相关的文献213篇,精读后筛选出85篇文献进行综述,见图2。
3.2 作者综述区别于他人他篇的特点 目前已有文章对外胚层中神经嵴来源的外胚层间充质干细胞进行综述,或是对外胚层间充质干细胞的特定治疗作用进行综述,缺少对外胚层间充质干细胞的整体综述。文章就外胚层来源的间充质干细胞相关研究进行广泛检索并作出总结,综述了已知的治疗作用机制,为外胚层间充质干细胞乃至干细胞治疗的研究提供研究思路。
3.3 综述的局限性 文章主要综述了外胚层间充质干细胞的主要来源以及一些重要应用进展。但是,目前外胚层间充质干细胞的研究较少,仅有少部分研究可以体现其外胚层组织修复的优越性,例如脊髓损伤修复和牙组织修复等。同时,外胚层间充质干细胞治疗的分子机制与信号通路虽有部分报道,但绝大多数治疗作用的机制尚未完全揭示。
3.4 综述的重要意义 文章总结了已有关于外胚层间充质干细胞获取与应用的研究,综述了部分外胚层间充质干细胞在治疗脊髓损伤与牙科疾病的作用机制,同时阐述了其在脓毒症治疗方面的新应用,为后续外胚层间充质干细胞在免疫微环境调控及其机理研究方面提供新方向,同时为揭示外胚层间充质干细胞治疗机制提供思路。
3.5 课题专家组继续开展外胚层间充质干细胞研究的想法 虽然对于外胚层间充质干细胞的研究已有很长时间,但是相关研究仍处于基础研究阶段。虽然研究显示外胚层间充质干细胞对于很多疾病具有治疗效果,但是治疗相关分子机制与信号通路研究仍未完全揭示。然而,外胚层间充质干细胞来源广泛,获取简便,损伤性低,对于神经系统疾病以及免疫疾病具有治疗作用,具有极佳的应用前景,为更好地应用于临床,后续应加强对其治疗机理及安全性的研究。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程

文题释义:
间充质干细胞:是干细胞的一种,具有亚全能分化潜能,在特定的体内外环境下能够诱导分化成为多种间质组织细胞。间充质干细胞具有干细胞的共性,即自我更新、多向分化和归巢的能力。
外胚层:外胚层系来源于卵细胞动物极附近的细胞浆,原肠胚形成时开始出现在胚的外表面或表面,最终分化成表皮与神经系统。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
间充质干细胞广泛存在于人体的多种组织与器官中,在机体的修复以及免疫调控中起到关键作用。目前为止,已有超过1 400项临床试验使用间充质干细胞,其适用领域包括骨质疾病、糖尿病、心血管疾病、自身免疫病及神经系统疾病等。目前研究较多的间充质干细胞主要来源于中胚层组织如骨髓、脂肪和脐带等,而对于外胚层组织少有涉及。值得一提的是,随着研究的深入,人们已经认识到外胚层间充质干细胞的诸多优点,其应用范围也日趋拓宽,使得其研究价值不可忽视,近年来对其的研究也在逐渐增多。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||