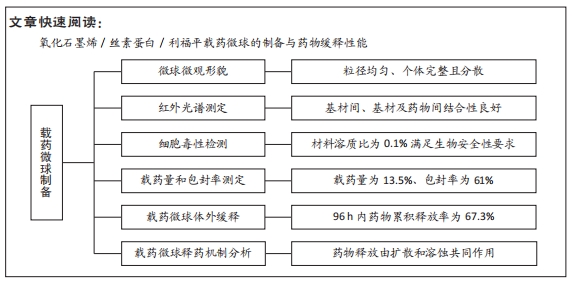
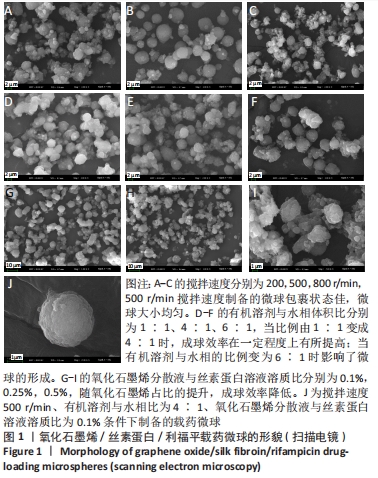
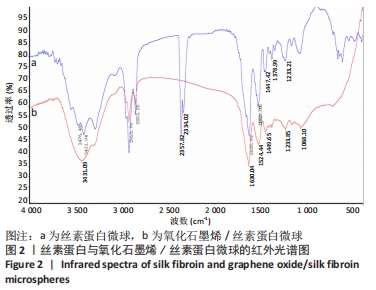
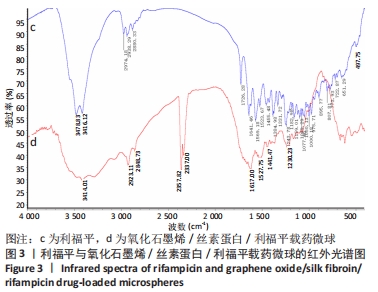
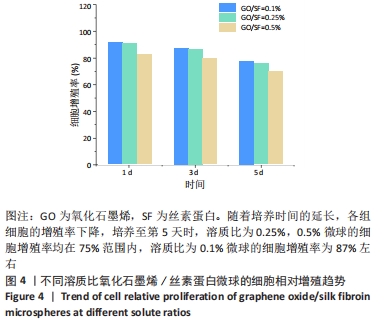
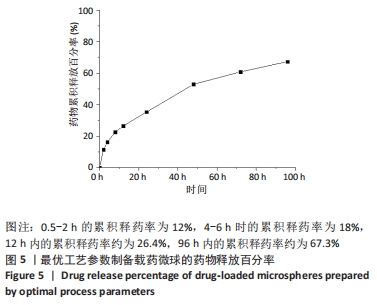
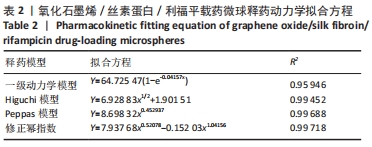
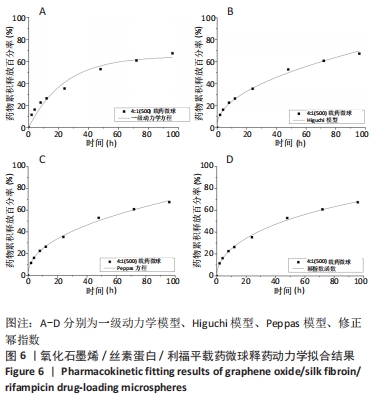
[1] 李大伟,马远征.骨关节结核局部药物缓释材料研究进展[J].中国防痨杂志,2013,35(5):376-378.
[2] 韩建军,宋金龙,谢印法,等.载药微球治疗恶性肝脏肿瘤的全程管理[J].中华介入放射学电子杂志,2020,2(7):7-16.
[3] 沈俊海,张金敏,全微雷,等.新型磁性氧化石墨烯的制备及其载药性能[J].中国科学,2016,46(8):800-809.
[4] 章美明.磁性氧化石墨烯基非共价修饰纳米复合材料的制备及其在药物递送方面的应用[D].镇江:江苏大学,2019.
[5] 雷海琳.氧化石墨烯基非共价修饰纳米复合材料的制备及其在药物递送方面的应用[D].镇江:江苏大学,2017.
[6] 沈贺,张立明,张智军.石墨烯在生物医学领域的应用[J].东南大学学报(医学版),2011,30(1):218-223.
[7] 王晨,许军,刘燕华,等.功能化氧化石墨烯作为药物载体材料的研究进展[J].中国药科大学学报,2017,48(1):117-124.
[8] KARKI N, TIWARI H, PAL M, et al. Functionalized graphene oxides for drug loading, release and delivery of poorly water soluble anticancer drug: A comparative study. Colloids Surf B Biointerfaces. 2018;169: 265-272.
[9] LIU Z, ROBINSON JT, SUN X, et al. PEGylated Nano-Graphene Oxide for Delivery of Water Insoluble Cancer Drugs. J Am Chem Soc. 2008; 130(33):10876-10877.
[10] 杨琳,刘晓松,孙建立,等.氧化石墨纳米颗粒吸附的5-氟尿嘧啶、抗体和细胞因子的体外生物活性研究和细胞因子的体外生物活性研究[J].肿瘤防治研究,2014,41(4):340-344.
[11] PEREZ-RIGUEIRO J, BIANCOTTO L, CORSINI P, et al. Supramolecular organization of regenerated silkworm silk fibroin bers. Int J Biol Macromol. 2009;44(2):195-202.
[12] KUNDU B, RAJKHOWA R, KUNDU SC, et al. Silk fibroin biomaterials for tissue regenerations. Adv Drug Deliv Rev. 2013;65(4):457-470.
[13] 刘纯,金梦瑶,张学农,等.双氯芬酸钠丝素蛋白-壳聚糖缓释微球的制备及体内外评价[J].中国药学杂志,2009,21(3):1642-1647.
[14] SUBIA B, KUNDU SC. Drug loading and release on tumor cells using silk fibroin-albumin nanoparticles as carriers. Nanotechnology. 2013; 24(3):1-11.
[15] 张云海.丝素蛋白药物缓释载体的制备及其性能研究[D].北京:北京理工大学,2015.
[16] 马永海.异烟肼、利福平缓释微球的制备及体内外释药性能的研究[D].宁夏:宁夏医科大学,2019.
[17] SUDIMACK J, LEE L. Targeted drug delivery via the folate receptor. Adv Drug Deliv Rev. 2000;41:147-162.
[18] LIU J, YAN L, WEI L, et al. Controlled-release neurotensin-loaded silk fibroin dressings improve wound healing in diabetic rat model. Bioact Mater. 2019;4:151-159.
[19] CROMMELIN DJA, FLORENCE AT. Towards more effective advanced drug delivery systems. Int J Pharm. 2013;454:496-511.
[20] 陈建辉.具有靶向标记功能的抗肿瘤药物缓释载体的制备及其研究[D].广州:华南理工大学,2018.
[21] WANG Z, NIU H, LI Z, et al. Superselective arterial embolization with drug-loaded microspheres for the treatment of unresectable breast cancer. Gland Surg. 2019;8(6):740-747.
[22] 刘亚珍,邱晓明,李松凯.正交设计优化万古霉素/聚乳酸-羟基乙酸共聚物微球的制备及体外药物释放[J].中国组织工程研究, 2019,23(2):211-217.
[23] 胡玉婷,李培源.氧化石墨烯作为药物载体的研究进展[J].山东化工,2019,48(24):50-56.
[24] 曾鹏,朱雪芬,操江飞,等.氧化石墨烯的制备及其对大肠埃希菌的抑菌性能研究[J].山东化工,2019,46(2):30-31.
[25] CHENG HKF, SAHOO NG, TAN YP, et al. Poly(vinyl alcohol) Nanocomposites Filled with Poly(vinyl alcohol)-Grafted Graphene Oxide. ACS Appl Mater Interfaces. 2012;4(5):2387-94.
[26] 钱文昊,苏俭生.纳米氧化石墨烯控释载药体系研究现状[J].中国临床医学,2016,23(3):383-387.
[27] 王亚茹,张青,雷芳,等.丝素蛋白-氧化石墨烯多孔微球支架的制备及性能测试[J].蚕业科学,2019,45(2):231-236.
[28] LI S, XIAO L, DENG H, et al. Remote controlled drug release from multi-functional Fe3O4/GO/Chitosan microspheres fabricated by an electrospray method. Colloids Surf B Biointerfaces. 2017;151:354-362.
[29] 熊龑,徐涛,龚良国.CCK-8法测定纤维蛋白胶对人脂肪干细胞的细胞毒性[J].中国当代医药,2019,26(18):38-40.
[30] 冯华龙,何升华,黄飞强. CCK-8法定量检测亚甲蓝对人髓核细胞的毒性[J].中国组织工程研究,2018,22(16):2532-2536.
[31] SINGH B, CHAUHAN GS, SHARMA DK, et al. The release dynamics of model drugs from the psylium and N-hydroxym-ethylacrylamide based hydrogels. Int J Pharm. 2006;325:15-25. |