中国组织工程研究 ›› 2022, Vol. 26 ›› Issue (4): 535-539.doi: 10.12307/2022.088
• 药物控释材料 drug delivery materials • 上一篇 下一篇
阿托伐他汀钙缓释微球制备方法的优化
刘童斌1,林 鹏2,张晓明1,董西玲1,曹 飞1,王 乐1,郭新星2
- 1滨州医学院附属医院口腔修复科,山东省滨州市 256600;2济南市口腔医院口腔正畸科,山东省济南市 250000
Optimization of preparation method of atorvastatin calcium sustained-release microspheres
Liu Tongbin1, Lin Peng2, Zhang Xiaoming1, Dong Xiling1, Cao Fei1, Wang Le1, Guo Xinxing2
- 1Department of Prosthodontics, Binzhou Medical University Hospital, Binzhou 256600, Shandong Province, China; 2Department of Orthodontics, Jinan Stomatological Hospital, Jinan 250000, Shandong Province, China
摘要:
文题释义:
药物缓释剂:是将药物包裹于特定的缓释介质中,使药物可以按要求缓慢地从介质中释放的制剂。与普通药物制剂相比,药物缓释剂可调整药物的理化特性、增加药物的稳定性、降低药物的不良反应;临床上用药频率的减少能显著增加患者的依从性。
去溶剂法:是在牛血清白蛋白水溶液中缓慢加入脱溶剂物质(例如无水乙醇),由于牛血清白蛋白水溶性低,脱溶剂活性剂的加入导致蛋白分子脱水,从而致使蛋白分子构象改变,逐渐变得卷曲,最终成团。此时形成的蛋白颗粒是极不稳定的,用水分散后仍可以重新溶解。
背景:近年来研究发现,他汀类药物在调节骨代谢、修复骨细微结构、抑制炎症、促进细胞增殖、修复血管内皮、调节信号通路传导等多方面具有显著效果。
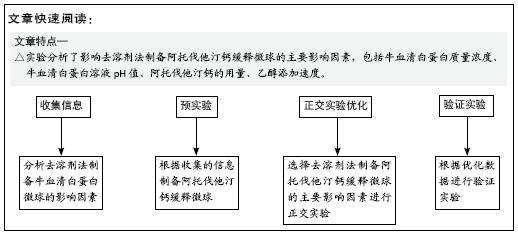
目的:对载阿托伐他汀钙缓释微球的制作参数进行优化,以期制备出载药量大、形态规则的缓释微球。
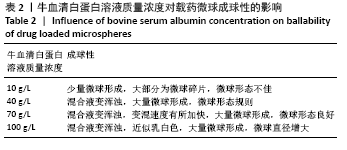
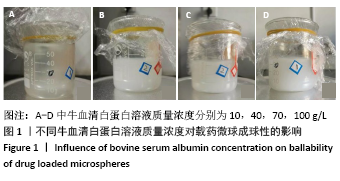
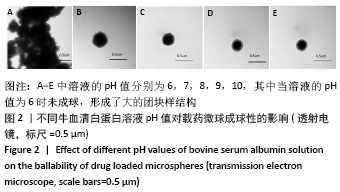
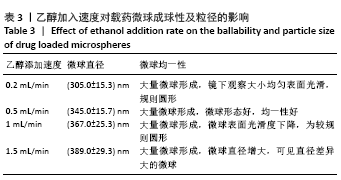
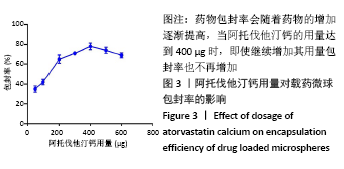
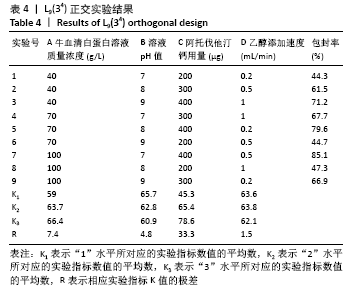
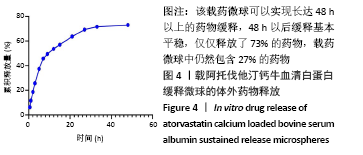
方法:采用去溶剂法制备载阿托伐他汀钙的牛血清白蛋白缓释微球,筛选出影响去溶剂过程的主要影响因素,包括血清白蛋白溶液质量浓度(40,70,100 g/L)及pH值(7,8,9)、阿托伐他汀钙的用量(200,300,400 µg)、乙醇添加速度(0.2,0.5,1 mL/min),通过正交实验筛选出最佳包封率的制备条件。在最佳组合工艺参数下制备载阿托伐他汀钙的牛血清白蛋白缓释微球,置于PBS中进行体外缓释性能测试。
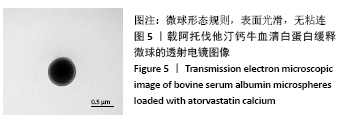
结果与结论:①最佳的制备工艺参数是:牛血清白蛋白溶液质量浓度为100 g/L、pH值为7,阿托伐他汀钙用量为400 µg,乙醇添加速度为0.2 mL/min;②在此参数下制备的微球形态规则,表面光滑,微球直径(425.0±13.8) nm,药物包封率高达85.70%,体外释放时间可持续 48 h以上,累积释放量达到73%,具有较为良好的缓释效果;③实验成功制备了负载阿托伐他汀钙的牛血清白蛋白缓释微球,此药物缓释微球具有较高载药量及稳定性,并可以实现的药物缓释。
https://orcid.org/0000-0003-3316-061X (王乐)
中国组织工程研究杂志出版内容重点:生物材料;骨生物材料; 口腔生物材料; 纳米材料; 缓释材料; 材料相容性;组织工程
中图分类号: