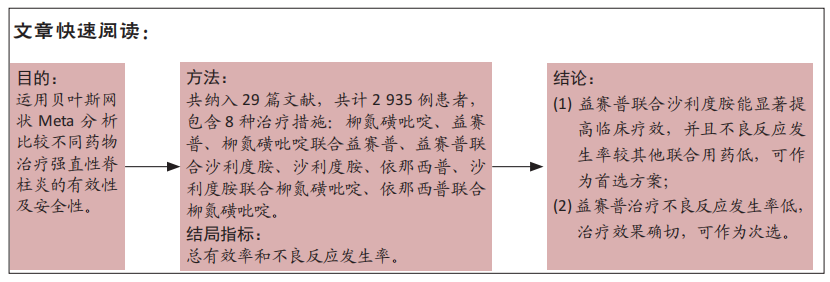
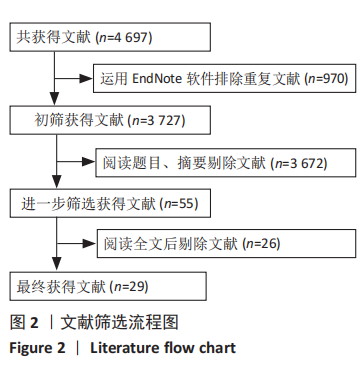
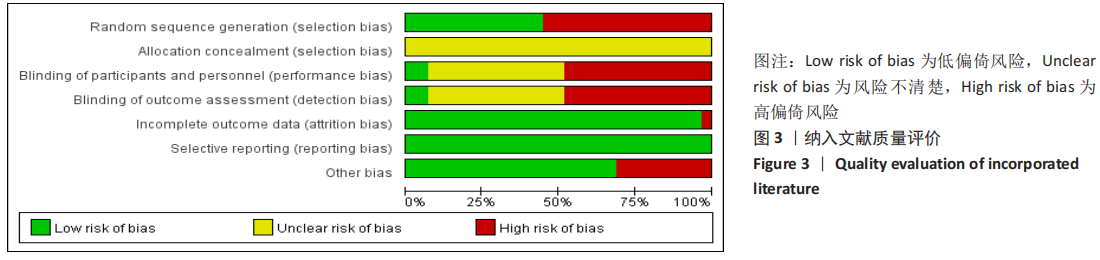
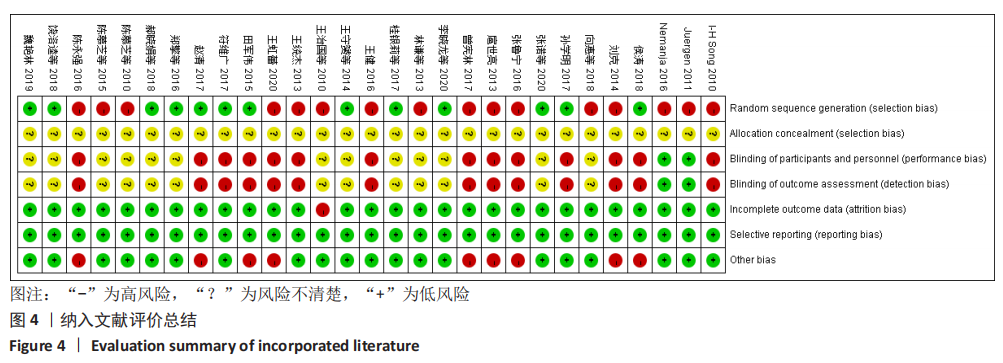
[1] 王勤俭,王燕.苁蓉独活散联合温针灸治疗肾阳亏虚型强直性脊柱炎的临床观察[J].中国实验方剂学杂志,2020,26(12):126-132.
[2] 孙学明,刘磊,曹君君,等.沙利度胺联合柳氮磺吡啶治疗强直性脊柱炎的疗效观察[J].药物评价研究,2017,40(10):1477-1480.
[3] 罗绮雯,陈国强,张红卫,等.依那西普注射剂联合雷公藤多苷片治疗强直性脊柱炎的临床研究[J].中国临床药理学杂志,2019, 35(15):1578-1580.
[4] BECCIOLINI A, BIGGIOGGERO M, FAVALLI EG. The role of methotrexate as combination therapy with etanerce in rheumatoid arthritis:retrospective analysis of a local registry. J Int Med Res. 2016;44(1 Suppl):113-118.
[5] 谢等花.强直性脊柱炎患者接受沙利度胺治疗的效果探讨[J].基层医学论坛,2020, 24(17):2405-2406.
[6] 李朝霞,曾珊,吴会霞,等.沙利度胺治疗强直性脊柱炎的安全性Meta分析[J].今日药学,2019,29(6):420-426.
[7] 张笑,罗晓舟,曹秋雨,等.针灸与柳氮磺胺吡啶治疗强直性脊柱炎有效性的网状Meta分析[J].中华中医药学刊,2018,36(10): 2321-2324.
[8] 刘志燕,柳芳,李朋梅,等.白芍总苷胶囊联合柳氮磺吡啶片对比柳氮磺吡啶片治疗强直性脊柱炎的疗效和安全性的Meta分析[J].中国药房,2018,29(17):2416-2420.
[9] 邢伟鹏,李无阴,侯宏理,等.沙利度胺治疗强直性脊柱炎疗效的Meta分析[J].中国药房,2018,29(1):116-120.
[10] 李晓群,李靖,都建苹.益赛普联合传统抗风湿药物治疗强直性脊柱炎的Meta分析[J].黑龙江医学,2018,42(1):93-96.
[11] 刘永军,戎红波.英夫利昔单抗治疗强直性脊柱炎疗效与安全性的Meta分析[J].中国新药与临床杂志,2016,35(11):815-821.
[12] 翟佳羽,吕青,赵敏菁,等.甲氨蝶呤和柳氮磺吡啶治疗强直性脊柱炎安全性和有效性的Meta分析[J].中山大学学报(医学科学版),2015,36(1):42-54.
[13] 侯东杰,王建民.益赛普与柳氮磺胺吡啶治疗强直性脊柱炎的Mete分析[J].宁夏医科大学学报,2014,36(5):545-552.
[14] VAN DER LINDEN S, VALKENBURG HA, CATS A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27(4): 361-368.
[15] 王统杰,王海琨.两种方法治疗活动期强直性脊柱炎的疗效比较[J].中国医药指南, 2013,11(27):205-207.
[16] 林谦,伍俊妍.注射用重组人Ⅱ型肿瘤坏死因子受体-抗体融合蛋白治疗强直性脊柱炎60例的临床观察[J].中国当代医药,2013, 20(21):87-88.
[17] 王守赟,阮文礼,宋子缨.注射用重组人Ⅱ型肿瘤坏死因子受体-抗体融合蛋白联合柳氮磺吡啶治疗强直性脊柱炎的临床观察[J].中国医院用药评价与分析,2014,14(12): 1083-1085.
[18] 扈世亮.益赛普联合沙利度胺用于难治性强直性脊柱炎的疗效研究[J].中国医学创新, 2013,10(22):32-33.
[19] 侯涛.沙利度胺治疗强直性脊柱炎的效果分析[J].河南医学研究,2018,27(4):662-663.
[20] 郝晓娟,赵花妮,张强,等.注射用重组Ⅱ型肿瘤坏死因子受体抗体融合蛋白联合柳氮磺吡啶治疗活动性强直性脊柱炎的疗效[J].临床医学研究与实践,2018,3(17):16-17.
[21] 桂银莉,史丽璞,郇稳,等.沙利度胺治疗强直性脊柱炎有效性与安全性的观察[J].现代诊断与治疗,2017,28(18):3382-3384.
[22] 符维广,李浩鹏.重组人Ⅱ型肿瘤坏死因子受体-抗体融合蛋白治疗强直性脊柱炎的临床效果[J].中国医药导报,2017,14(6):112-115.
[23] 陈永强.益赛普组合柳氮磺吡啶在强直性脊柱炎中的疗效观察[J].北方药学,2016, 13(11):11-12.
[24] 陈慕芝,照日格图,王海云,等.重组人Ⅱ型肿瘤坏死因子受体-抗体融合蛋白与传统免疫抑制剂治疗强直性脊柱炎的临床对照研究[J].新疆医科大学学报,2010,33(8):913-915.
[25] 陈慕芝,孙学斌,段红妍.依那西普与柳氮磺吡啶治疗强直性脊柱炎的效果[J].中国医药导报,2015,12(7):82-85.
[26] 张鲁宁.益赛普治疗难治性强直性脊柱炎患者的疗效观察[J].中国民康医学,2016, 28(10):34-35.
[27] 魏艳林,夏楠楠,张伟峰,等.沙利度胺联合柳氮磺吡啶对强直性脊柱炎患者炎性反应的影响[J].临床医学工程,2019,26(7):957-958.
[28] 曾宪林.沙利度胺治疗强直性脊柱炎的长期疗效与安全性分析[J].齐齐哈尔医学院学报,2017,38(20):2412-2414.
[29] 王健,吉健华,王医林,等.沙利度胺与柳氮磺吡啶治疗强直性脊柱炎效果比较观察[J].人民军医,2016,59(1):45-47.
[30] 刘克.依那西普联合柳氮磺吡啶治疗强直性脊柱炎的临床疗效[J].中国医药指南,2014, 12(36):217-218.
[31] 孙学明,刘磊,曹君君,等.沙利度胺联合柳氮磺吡啶治疗强直性脊柱炎的疗效观察[J].药物评价研究,2017,40(10):1477-1480.
[32] 郑擎,徐鸣俊.重组人Ⅱ型肿瘤坏死因子受体抗体融合蛋白治疗强直性脊柱炎的临床研究[J].中国临床药理学杂志,2016,32(17): 1561-1564.
[33] 饶洛逵,陆熙宴.依那西普联合柳氮磺吡啶治疗强直性脊柱炎的疗效及安全性[J].中国医院用药评价与分析,2018,18(3):351-353.
[34] 田军伟.沙利度胺治疗强直性脊柱炎临床效果观察[J].河南医学研究,2015,24(9):43-45.
[35] 张诺,郭明蔚,陈桂武,等.益赛普联合柳氮磺吡啶治疗强直性脊柱炎疗效及miR-29a、miR-146a的变化[J].分子诊断与治疗杂志,2020,12(5):587-591.
[36] 向惠,衡明强.沙利度胺联合注射用重组人Ⅱ型肿瘤坏死因子受体-抗体融合蛋白对难治性强直性脊柱炎的治疗效果[J].中国继续医学教育,2018,10(19):127-128.
[37] 赵清.益赛普联合沙利度胺治疗难治性强直性脊柱炎临床研究[J].中国药业,2017,26(9): 47-49.
[38] 王治国,佟胜全,饶莉,等.短期益赛普联合柳氮磺胺吡啶治疗强直性脊柱炎的疗效研究[J].实用临床医药杂志,2010,14(7):65-66.
[39] 王虹蕾.益赛普治疗强直性脊柱炎的疗效[J].中国实用医刊,2012,39(15):122.
[40] 李晓龙,苏振炎,张益宏,等.注射用重组人Ⅱ型肿瘤坏死因子受体-抗体融合蛋白联合沙利度胺对强直性脊柱炎患者血清炎性因子的影响[J].颈腰痛杂志,2020,41(3):321-324.
[41] BRAUN J, VAN DER HORST-BRUINSMA IE, HUANG F, et al. Clinical efficacy and safety of etanercept versus sulfasalazine in patients with ankylosing spondylitis: a randomized, double-blind trial. Arthritis Rheum. 2011;63(6):1543-1551.
[42] SONG IH, HERMANN KG, HAIBEL H, et al. Relationship between active inflammatory lesions in the spine and sacroiliac joints and new development of chronic lesions on whole-body MRI in early axial spondyloarthritis: results of the ESTHER trial at week 48. Ann Rheum Dis. 2011;70(7):1257-1263.
[43] DAMJANOV N, SHEHHI WA, HUANG F, et al. Assessment of clinical efficacy and safety in a randomized double-blind study of etanercept and sulfasalazine in patients with ankylosing spondylitis from Eastern/Central Europe, Latin America, and Asia. 2016;36(5):643-651.
[44] SCHULZ M, DOTZLAW H, NEECK G. Ankylosing spon-dylitis and rheumatoid arthritis: serum levels of TNF-αand its soluble receptors during the course of therapy withetanercept and infliximab. Biomed Res Int. 2014;2014:675108.
[45] 石向慧,纪禄风,陈蓓蓓,等.白细胞介素-6在强直性脊柱炎诊治中的研究现状[J].风湿病与关节炎,2017,6(4):65-69.
[46] PARHAM C, CHIRICA M, TIMANS J, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168(11):5699-5708.
[47] 曹芳,汪悦.中医健脾法与IL-23/Th17/IL-17通路在强直性脊柱炎中的研究进展[J].辽宁中医杂志,2017,44(3):662-665.
[48] 王星,张莹,郑军,等.TNF-α拮抗剂治疗早期强直性脊柱炎临床效果观察[J].西南国防医药,2019,29(12):1200-1203.
[49] CHOUDHURY MR, HASSAN MM, KABIR ME, et al. An open label clinical trial of Thalidomide in NSAIDs refractory ankylosing spondylitis. Mod Rheumatol. 2018;28(4):730-732.
[50] 吕艳霞.沙利度胺联合重组人肿瘤坏死因子受体-抗体融合蛋白治疗强直性脊柱炎50例临床观察[J].中国药物与临床,2014, 14(12):1712-1713.
[51] 陈伟伦,姚翠霞,林永明.沙利度胺联合益赛普治疗难治性强直性脊柱炎临床探析[J].中国医药科学,2013,24(2):92-93.
[52] VAN DARTEL SA, FRANSEN J, KIEVIT W, et al. Difference in the risk of serious infections in patients with rheumatoid arthritis treated with adalimumab, infliximab and etanercept:results from the Dutch Rheumatoid Arthritis Monitoring (DREAM) registry. Ann Rheum Dis. 2013;72(6):895-900.
[53] ALI T, KAITHA S, MAHMOOD S, et al. Clinical use of anti-TNF therapy and increased risk of infections. Drug Healthc Patient Saf. 2013;5:79-99.
[54] NAVARRA S V, TANG B, LU L, et al. Risk of tuberculosis with anti-tumor necrosis factor-α therapy: substantially higher number of patients at risk in Asia. Int J Rheum Dis. 2014; 17(3):291-298.
[55] DREYER L, MELLEMKJæ RL, ANDERSEN AR, et al. Incidences of overall and site specific cancers in TNFα inhibitor treated patients with rheumatoid arthritis and other arthritides-a follow-up study from the DANBIO Registry. Ann Rheum Dis. 2013;72(1):79-82.
[56] 汪洋,马民玉.风湿祛痛胶囊联合柳氮磺吡啶治疗强直性脊柱炎的临床研究[J].现代药物与临床,2020,35(10):2002-2005.
[57] HAROON N, INMAN RD, LEARCH TJ, et al. The impact of tumor necrosis factorαinhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum. 2013;65:2645-2654.
[58] 陈庆花,杨永红,邱悦群,等.益赛普治疗强直性脊柱炎有效性和安全性临床对比研究[J].中国疼痛医学杂志,2011,17(12):760-761.
[59] 刘维,张迪,吴沅皞,等.中药联合重组人Ⅱ型肿瘤坏死因子受体-抗体融合蛋白治疗强直性脊柱炎疗效观察[J].中国中西医结合杂志,2016,36(6):663-667.
[60] 胡久丽,朱孝芹,肖旭,等.沙利度胺致严重药品不良反应2例[J].中国现代应用药学, 2019,36(18):2334-2335.
[61] 陈锦敏,柳鹏程,余正.美国FDA药品上市后风险管理措施研究及对我国的启示-以沙利度胺为例[J].中国新药杂志,2020,29(23): 2654-2659.
(责任编辑:WJ,ZN,ZH)
|