Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (34): 5455-5461.doi: 10.12307/2023.840
Previous Articles Next Articles
Regulation of interleukin-4 on osteoclast differentiation during bone regeneration guided by bone replacement materials
Li Li1, Li Xiao2, Li Duchenhui1, Zhang Jie1, 3, Xiao Tianjiao1, Kang Jiabing1, Tian Ai1
- 1School of Stomatology, Guizhou Medical University/Affiliated Stomatological Hospital, Guiyang 550004, Guizhou Province, China; 2Guiyang Stomatological Hospital, Guiyang 550002, Guizhou Province, China; 3The First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine, Guiyang 550001, Guizhou Province, China
-
Received:2022-10-21Accepted:2022-12-12Online:2023-12-08Published:2023-04-20 -
Contact:Tian Ai, Associate professor, Associate chief physician, Master’s supervisor, School of Stomatology, Guizhou Medical University/Affiliated Stomatological Hospital, Guiyang 550004, Guizhou Province, China -
About author:Li Li, Master candidate, School of Stomatology, Guizhou Medical University/Affiliated Stomatological Hospital, Guiyang 550004, Guizhou Province, China -
Supported by:National Natural Science Foundation of China, No. 81760192, 82260193 (to TA)
CLC Number:
Cite this article
Li Li, Li Xiao, Li Duchenhui, Zhang Jie, Xiao Tianjiao, Kang Jiabing, Tian Ai. Regulation of interleukin-4 on osteoclast differentiation during bone regeneration guided by bone replacement materials[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5455-5461.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

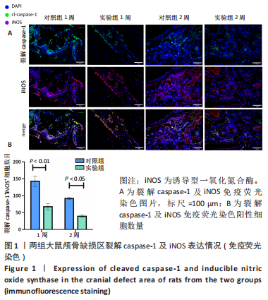
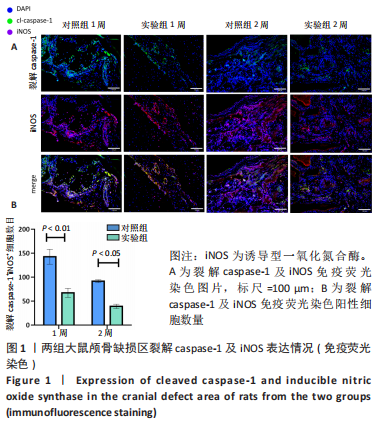
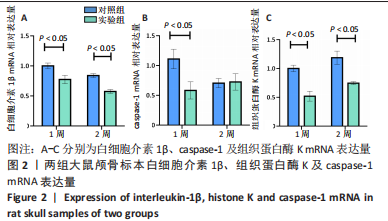
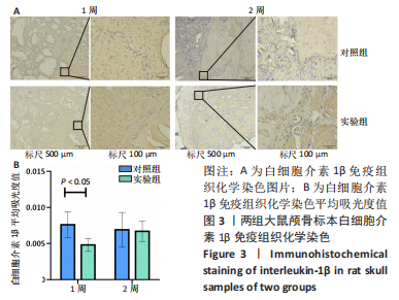
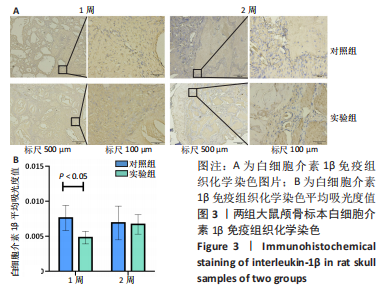
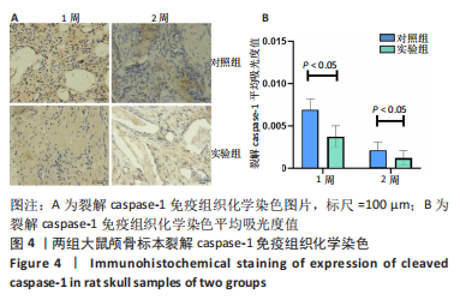
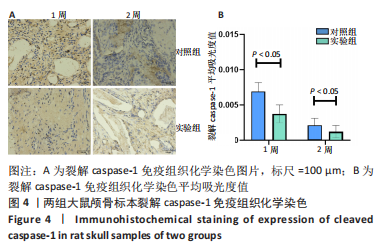
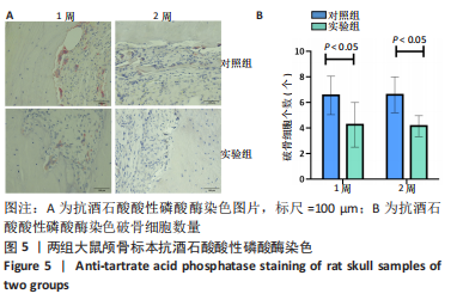
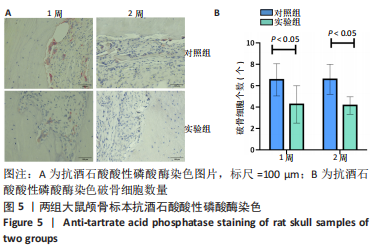
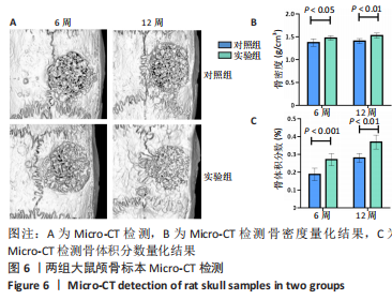
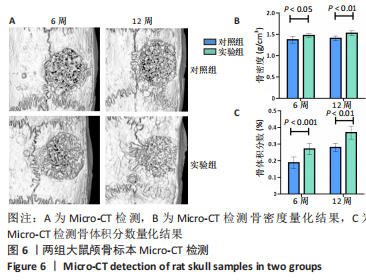
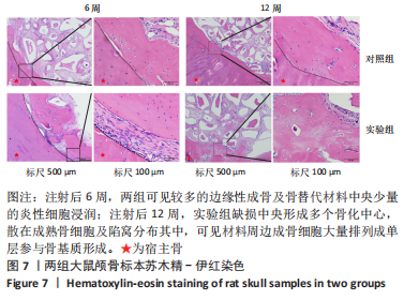
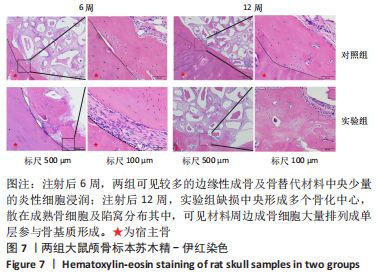
利用 Micro-CT检测及苏木精-伊红染色观察两组大鼠颅骨成骨情况。Micro-CT检测显示,注射后6周,两组均可见大面积骨粉颗粒,骨缺损区边缘部可见少量成骨,实验组边缘新生骨量较对照组明显。注射后12周,两组中央缺损区仍然见骨粉颗粒影,边缘成骨较6周明显,实验组边缘成骨较对照组明显,骨质与正常骨质接近。分析参数结果显示,实验组注射后6,12周的骨体积分数、骨密度值均显著高于对照组(P < 0.05,P < 0.01,P < 0.001)。 苏木精-伊红染色结果显示,注射后6周,两组可见较多的边缘性成骨及骨替代材料中央少量的炎性细胞浸润;注射后12周,实验组缺损中央形成多个骨化中心,散在成熟骨细胞及陷窝分布其中,可见材料周边成骨细胞大量排列成单层参与骨基质形成。"

| [1] MADDALONE M, MIRABELLI L, VENINO PM, et al. Long-term stability of autologous bone graft of intraoral origin after lateral sinus floor elevation with simultaneous implant . Clin Implant Dent Relat Res. 2018;20(5):713-721. [2] LA MONACA G, IEZZI G, CRISTALLI MP, et al. Comparative Histological and Histomorphometric Results of Six Biomaterials Used in Two-Stage Maxillary Sinus Augmentation Model after 6-Month Healing. Biomed Res Int. 2018;2018: 9430989. [3] CORBELLA S, TASCHIERI S, DEL FABBRO M. Long-term outcomes for the treatment of atrophic posterior maxilla: a systematic review of literature. Clin Implant Dent Relat Res. 2015;17(1):120-132. [4] TRINDADE R, ALBREKTSSON T, TENGVALL P, et al. Foreign Body Reaction to Biomaterials: On Mechanisms for Buildup and Breakdown of Osseointegration. Clin Implant Dent Relat Res. 2016;18(1):192-203. [5] KLOPFLEISCH R, JUNG F. The pathology of the foreign body reaction against biomaterials. J Biomed Mater Res A. 2017;105(3):927-940. [6] SHEIKH Z, BROOKS PJ, BARZILAY O, et al. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials (Basel). 2015; 8(9):5671-5701. [7] BANDYOPADHYAY A, SHIVARAM A, TARAFDER S, et al. In Vivo Response of Laser Processed Porous Titanium Implants for Load-Bearing Implants. Ann Biomed Eng. 2017;45(1):249-260. [8] KAMITAKAHARA M, TATSUKAWA E, SHIBATA Y, et al. Effect of silicate incorporation on in vivo responses of α-tricalcium phosphate ceramics. J Mater Sci Mater Med. 2016;27(5):97. [9] FERNANDEZ-YAGUE MA, ABBAH SA, MCNAMARA L, et al. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv Drug Deliv Rev. 2015;84:1-29. [10] GRUBER R. Osteoimmunology: Inflammatory osteolysis and regeneration of the alveolar bone. J Clin Periodontol. 2019; 46 Suppl 21:52-69. [11] ZHENG ZW, CHEN YH, WU DY, et al. Development of an Accurate and Proactive Immunomodulatory Strategy to Improve Bone Substitute Material-Mediated Osteogenesis and Angiogenesis. Theranostics. 2018; 8(19):5482-5500. [12] VISHWAKARMA A, BHISE NS, EVANGELISTA MB, et al. Engineering Immunomodulatory Biomaterials To Tune the Inflammatory Response. Trends Biotechnol. 2016;34(6):470-482. [13] MURRAY PJ. Macrophage Polarization. Annu Rev Physiol. 2017;79:541-566. [14] YUNNA C, MENGRU H, LEI W, et al. Macrophage M1/M2 polarization. Eur J Pharmacol.2020. 877:173090. [15] 黎良盛,翁一杰,魏劲松.NLRP3炎性小体在骨质疏松中的研究进展[J].海南医学,2022,33(15):2012-2016. [16] KESAVARDHANA S, KANNEGANTI T D. Mechanisms governing inflammasome activation, assembly and pyroptosis induction. Int Immunol. 2017;29(5):201-210. [17] YOUM YH, GRANT RW, MCCABE LR, et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 2013;18(4):519-532. [18] ROCHA FRG, DELITTO AE, DE SOUZA JAC, et al. Relevance of Caspase-1 and Nlrp3 Inflammasome on Inflammatory Bone Resorption in A Murine Model of Periodontitis. Sci Rep. 2020;10(1):7823. [19] CHEN Y, YANG Q, LV C, et al. NLRP3 regulates alveolar bone loss in ligature-induced periodontitis by promoting osteoclastic differentiation. Cell Prolif. 2021;54(2):e12973. [20] WANG Y, QI H, MIRON RJ, et al. Modulating macrophage polarization on titanium implant surface by poly(dopamine)-assisted immobilization of IL4. Clin Implant Dent Relat Res. 2019;21(5):977-986. [21] KOHNO Y, LIN T, PAJARINEN J, et al. Treating Titanium Particle-Induced Inflammation with Genetically Modified NF-κB Sensing IL-4 Secreting or Preconditioned Mesenchymal Stem Cells in Vitro. ACS Biomater Sci Eng. 2019; 5(6):3032-3038. [22] BOSCH C, MELSEN B, VARGERVIK K. Importance of the critical-size bone defect in testing bone-regenerating materials. J Craniofac Surg, 1998; 9(4):310-316. [23] VAJGEL A, MARDAS N, FARIAS BC, et al. A systematic review on the critical size defect model. Clin Oral Implants Res. 2014;25(8):879-893. [24] EE JWY, BANCE ML. Physiology of Osseointegration. Otolaryngol Clin North Am. 2019;52(2):231-242. [25] MARTINEZ F, GORDON S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. [26] SICA A, MANTOVANI A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787-795. [27] IVASHKIV LB. Epigenetic regulation of macrophage polarization and function. Trends Immunol. 2013;34(5):216-223. [28] KAO WJ, MCNALLY AK, HILTNER A, et al. Role for interleukin-4 in foreign-body giant cell formation on a poly(etherurethane urea) in vivo. J Biomed Mater Res. 1995;29(10):1267-1275. [29] MARIANI E, LISIGNOLI G, BORZì RM, et al. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int J Mol Sci. 2019;20(3):636. [30] 赵月鑫,陈滨.巨噬细胞极化在骨组织工程免疫研究中的进展[J].中国组织工程研究,2022,26(13):2120-2126. [31] ZHAO DW, ZUO KQ, WANG K, et al. Interleukin-4 assisted calcium-strontium-zinc-phosphate coating induces controllable macrophage polarization and promotes osseointegration on titanium implant. Mater Sci Eng C Mater Biol Appl. 2021;118:111512. [32] MARSELL R, EINHORN TA. The biology of fracture healing. Injury. 2011;42(6):551-555. [33] BANK RA, ZANDSTRA J, ROOM H, et al. Biomaterial Encapsulation Is Enhanced in the Early Stages of the Foreign Body Reaction During Conditional Macrophage Depletion in Transgenic Macrophage Fas-Induced Apoptosis Mice. Tissue Eng Part A. 2017;23(19-20):1078-1087. [34] LIN T, PAJARINEN J, KOHNO Y, et al. Transplanted interleukin-4--secreting mesenchymal stromal cells show extended survival and increased bone mineral density in the murine femur. Cytotherapy. 2018;20(8):1028-1036. [35] LIU J, CAO X. Cellular and molecular regulation of innate . Cell Mol Immunol. 2016;13(6):711-721. [36] 李骁,唐正龙,田艾.免疫调节在骨组织愈合中的研究[J].重庆医学,2021, 50(18):3225-3229. [37] HANEKLAUS M, O’NEILL LA. NLRP3 at the interface of metabolism and inflammation. Immunol Rev. 2015;265(1):53-62. [38] MURAKAMI T, NAKAMINAMI Y, TAKAHATA Y, et al. Activation and Function of NLRP3 Inflammasome in Bone and Joint-Related Diseases. Int J Mol Sci. 2022; 23(10):5365. [39] ZHANG J, LIU X, WAN C, et al. NLRP3 inflammasome mediates M1 macrophage polarization and IL-1β production in inflammatory root resorption. J Clin Periodontol. 2020;47(4):451-460. [40] LU J, XIE S, DENG Y, et al. Blocking the NLRP3 inflammasome reduces osteogenic calcification and M1 macrophage polarization in a mouse model of calcified aortic valve stenosis. Atherosclerosis. 2022;347:28-38. [41] ZANG Y, SONG JH, OH SH, et al. Targeting NLRP3 Inflammasome Reduces Age-Related Experimental Alveolar Bone Loss. J Dent Res. 2020;99(11):1287-1295. [42] ZHANG Q, YU W, LEE S, et al. Bisphosphonate Induces Osteonecrosis of the Jaw in Diabetic Mice via NLRP3/Caspase-1-Dependent IL-1β Mechanism. J Bone Miner Res. 2015;30(12):2300-2312. [43] HACHIM D, LOPRESTI ST, YATES CC, et al. Shifts in macrophage phenotype at the biomaterial interface via IL-4 eluting coatings are associated with improved implant integration. Biomaterials. 2017;112:95-107. |
| [1] | Wen Xinghua, Ding Huanwen, Cheng Kai, Yan Xiaonan, Peng Yuanhao, Wang Yuning, Liu Kang, Zhang Huiwu. Three-dimensional finite element model analysis of intramedullary nailing fixation design for large femoral defects in Beagle dogs [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1371-1376. |
| [2] | Tang Haotian, Liao Rongdong, Tian Jing. Application and design of piezoelectric materials for bone defect repair [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1117-1125. |
| [3] | Xu Yan, Li Ping, Lai Chunhua, Zhu Peijun, Yang Shuo, Xu Shulan. Piezoelectric materials for vascularized bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1126-1132. |
| [4] | Yan Le, Zhang Huiping, Dai Lintong. Mesenchymal stem cells for allergic rhinitis: a meta-analysis based on animal experiments [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 977-984. |
| [5] | Zhang Min, Zhang Xiaoming, Liu Tongbin. Application potential of naringin in bone tissue regeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 787-792. |
| [6] | Chen Guodong, Zheng Meiyan, Zhang Peng, Wang Zhenchao, Jin Lixin. Changes in sensory neurons and astrocytes and the expression of interleukin 1beta and glial fibrillary acidic protein in the rat spinal cord after selective dorsal rhizotomy [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 726-731. |
| [7] | Liu Zixuan, Li Yan, Ji Lin, Xia Delin. Biological properties of nano-hydroxyapatite-zinc oxide composite scaffolds and their effects on the behavior of MC3T3-E1 osteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5441-5447. |
| [8] | Li Xinlun, Zhu Yushu, Yang Yiling, He Siqi, Wen Nan, Mu Yandong. Construction and in vivo osteogenesis of microspheres loaded with immunomodulatory peptide/miR-26a complexes for slow release [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5469-5476. |
| [9] | You Yan, Chen Jiawen, Lin Binbin, Wu Jingyi, Liu Peng, Wu Buling, Sun Tianyu. Mechanism and application of glycosaminoglycan in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5538-5545. |
| [10] | Zhao Mingyue, Yang Shun, Tu Xiling, Gao Li, Yang Kun, Liu Qi. Application of platelet-rich plasma combined with electrospun nanoscaffolds in bone and soft tissue [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5554-5560. |
| [11] | Tang Li, Pan Yao, Zhu Guochen. Epidermal neural crest stem cells of hair follicles regulate the local expression of inflammatory factors after facial nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(33): 5249-5255. |
| [12] | Xu Rongwei, Wang Hao, Fu Qiuyue, Lan Xingming, Yang Kun. Bidirectional interaction between inflammatory factors and dental pulp stem cells during bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(33): 5385-5393. |
| [13] | Shan Chao, Wu Zeyu, Zhao jin. Effects of hypoxia environment on microvessels and bone metabolism and bone repair in chronic periodontitis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(32): 5232-5237. |
| [14] | Song Meiling, Li Zhengyu, Ai Zizheng, Li Jingna, Zeng Qingfeng, Han Qianqian, Dong Xieping. Repair effect of different hydroxyapatite/beta-tricalcium phosphate coated scaffolds on bone defects [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(30): 4809-4816. |
| [15] | Liu Huan, Li Han, Ma Yunhao, Zhong Weijian, Ma Guowu. Osteogenic capacity of partially demineralized dentin particles in the maxillary sinus lift [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 354-359. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||