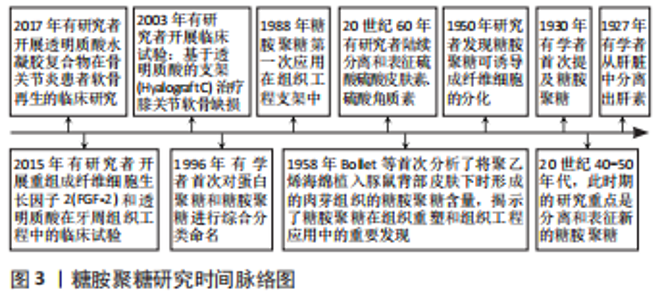
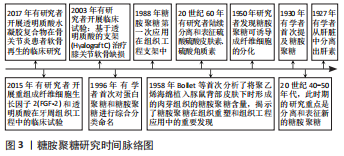
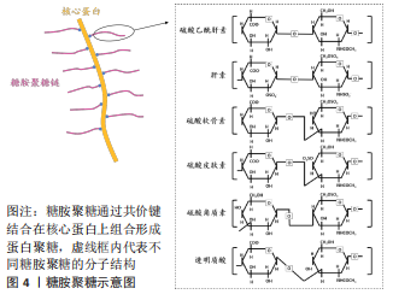
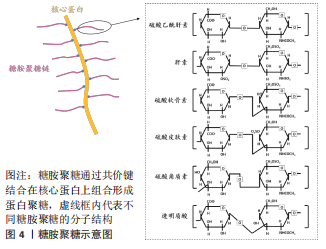
Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (34): 5538-5545.doi: 10.12307/2023.835
Previous Articles Next Articles
Mechanism and application of glycosaminoglycan in bone tissue engineering
You Yan1, Chen Jiawen1, Lin Binbin1, Wu Jingyi2, Liu Peng3, Wu Buling1, 4, Sun Tianyu5
- 1School of Stomatology, Southern Medical University, Guangzhou 510515, Guangdong Province, China; 2Center of Implant Dentistry, Stomatological Hospital, Southern Medical University, Guangzhou 510280, Guangdong Province, China; 3Zhujiang Hospital, Southern Medical University, Guangzhou 510282, Guangdong Province, China; 4Southern Medical University-Shenzhen Stomatology Hospital (Pingshan), Shenzhen 518118, Guangdong Province, China; 5Department of Periodontology, Stomatological Hospital, Southern Medical University, Guangzhou 510280, Guangdong Province, China
-
Received:2022-11-04Accepted:2022-12-03Online:2023-12-08Published:2023-04-22 -
Contact:Sun Tianyu, MD, Attending physician, Department of Periodontology, Stomatological Hospital, Southern Medical University, Guangzhou 510280, Guangdong Province, China -
About author:You Yan, Master candidate, School of Stomatology, Southern Medical University, Guangzhou 510515, Guangdong Province, China -
Supported by:the National Natural Science Foundation of China, No. 81900956 (to WJY); the Scientific Research and Cultivation Plan Project of Stomatological Hospital of Southern Medical University, No. PY2018036 (to STY); the Foundation for Basic and Applied Basic Research of Guangdong Province, No. 2020A1515110852 (to STY)
CLC Number:
Cite this article
You Yan, Chen Jiawen, Lin Binbin, Wu Jingyi, Liu Peng, Wu Buling, Sun Tianyu. Mechanism and application of glycosaminoglycan in bone tissue engineering[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5538-5545.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
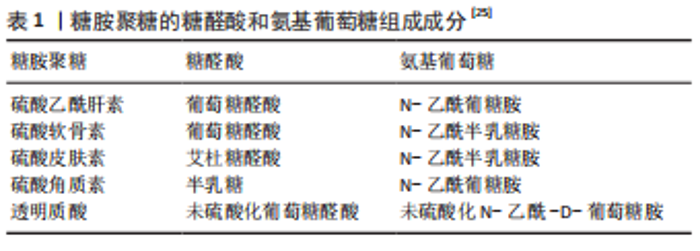
2.1 蛋白聚糖和糖胺聚糖的结构组成 蛋白聚糖是一类复杂的多糖大分子,由核心蛋白和一个或多个糖胺聚糖链通过四糖连接区共价连接组成[16],负责协调发育和组织稳态中的多种基本生物学功能,例如细胞外基质的形成、胚胎发育和组织更新等[17-18]。蛋白聚糖同样广泛参与了骨的形成,且其表达具有明显的时空特异性,在骨组织发育及修复中发挥必不可少的作用[19-25]。其中,结构多样的糖胺聚糖是蛋白聚糖具有丰富的生物学功能的结构基础。糖胺聚糖是一类含重复的二糖单元构成的直链酸性多糖大分子,其二糖单元主要含有氨基葡萄糖和糖醛酸(葡萄糖醛酸和/或艾杜糖醛酸)彼此之间通过糖苷键连接,见表1,以游离态存在或以附着于核心蛋白的形式存在于细胞表面和细胞外基质中,相对分子质量10 000-100 000[19]。糖胺聚糖的二糖单元结构中几乎都含-NHAc,-COOH,-OH和-SO3H 等基团,能够提供多种修饰位点 [20-21]。"
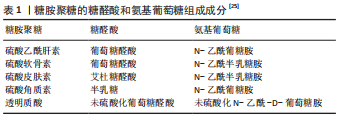
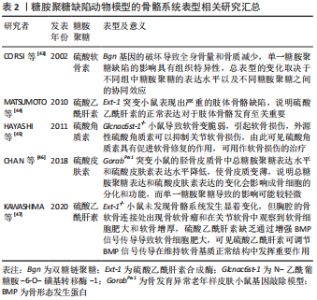
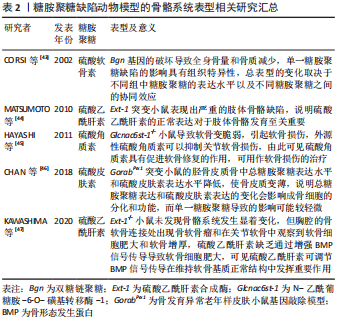
透明质酸是最简单的一类糖胺聚糖,结构均一且不经过硫酸化。与其他类型的糖胺聚糖相比,硫酸角质素是惟一的一个不含糖醛酸基和不通过四糖连接物连接到核心蛋白的糖胺聚糖,可进一步分为硫酸角质素Ⅰ、硫酸角质素Ⅱ和 硫酸角质素Ⅲ[27]。 糖胺聚糖和骨组织发育再生息息相关,既往研究指出多种参与编码糖胺聚糖生物合成修饰的基因突变会使其缺乏完整的结构和功能,从而导致遗传性骨发育疾病。有研究构建了糖基转移酶(Csgalnact1,Chst11)基因敲除小鼠模型,该酶是硫酸软骨素生物合成中的关键糖基转移酶之一,模型观察到小鼠颅颌面部发育异常和全身骨骼发育不良,具体表现为骨质矿化不足[28]。这说明硫酸软骨素的显著减少可导致膜内骨化发育不全,从而影响骨骼的形成。同时,糖胺聚糖的硫酸化模式也影响着骨组织正常发育过程的信号分子调控。磺基转移酶1负责催化转运硫酸盐修饰,其功能缺失同样可导致 Chst11 敲除小鼠全身软骨生长板的发育异常和骨骼畸形[29]。硫酸乙酰肝素 2-O-磺基转移酶 1是合成硫酸乙酰肝素的特异性酶之一,其变异或缺失会导致硫酸乙酰肝素合成异常,从而影响总体硫酸化模式,最终导致突变患者骨骼发育异常,表现为颅面四肢畸形、脊柱侧凸和骨组织成熟延迟等。研究者通过进一步分析突变患者的成纤维细胞,发现 N-6-硫酸化的增加和 2-O-硫酸化的缺乏使硫酸乙酰肝素的硫酸化模式异常,导致与成纤维细胞生长因子(fibroblast growth factor,FGF)-1 和 FGF-2 的亲和力显著降低,进而降低了 ERK1/2 的磷酸化水平,最终抑制了 MAPK 信号传导,减弱了成骨分化作用[30]。由此可见,糖胺聚糖结构的完整性和硫酸化功能修饰对骨组织发育起着关键的作用。 骨形成是长期的动态过程,糖胺聚糖的表达具有时空特异性,多种糖胺聚糖广泛参与细胞成骨分化所涉及的信号传导。一项体外研究对小鼠 MC3T3-E1 细胞成骨分化过程中的糖胺聚糖表达进行动态分析,发现糖胺聚糖合成酶及其含量随着分化过程逐渐上升[31]。除此之外,糖胺聚糖还以不同的方式参与成骨过程中分子信号的传导。在骨形成初期,骨缺损部位的早期血管化是至关重要的,硫酸乙酰肝素作为血管发育的关键调节剂,可以通过双重作用调节血管后期成熟和稳态过程。在 Ang-Tie 信号轴中,硫酸乙酰肝素可与 Ang 1 或 Ang 4 和 Tie 2 受体形成稳定三元复合物,调节该途径的下游通路,增强内皮细胞的生存信号。不仅如此,硫酸乙酰肝素还能与 Tie 1直接结合,促进并稳定Tie 1-Tie 2 异源二聚体的形成。GRIFFIN 等[32]通过 CRISPR-Cas9 技术构建硫酸乙酰肝素 Tie 1 敲除小鼠模型,实验模型表明硫酸乙酰肝素 Tie 1 复合物的破坏使 Tie1 和 Tie2 蛋白表达水平显著降低,干扰了 AKT 信号的通路传导,最终扰乱血管的成熟化。MURALI 等[33]发现硫酸乙酰肝素能促进骨形态发生蛋白2 和其受体的结合,同时抑制拮抗物头蛋白对骨形态发生蛋白通路的拮抗作用,触发 pSMAD 的激活,上调骨形态发生蛋白通路活性。最新研究指出硫酸盐基团是硫酸乙酰肝素与配体蛋白结合的亲和位点。N-硫酸化基团为骨形态发生蛋白2提供了关键的结合位点,并且是后续下游信号传导所必需的。N-硫酸盐基团的敲除显著减少了骨形态发生蛋白2与其受体的结合和 Smad 1/5/8 磷酸化减少,导致骨组织减少[34]。可见,硫酸乙酰肝素不但可以协助配体梯度扩散,影响与其相应受体之间的相互作用,还可以与配体受体形成复合物参与促进成骨分化的信号通路传导。 硫酸软骨素是构成骨基质最主要的糖胺聚糖之一,研究进一步发现硫酸软骨素能够为信号蛋白、生长因子提供结合位点,并与之特异性结合,对细胞的成骨分化以及基质的矿化能力具有正向作用[35]。当对 MC3T3-E1 细胞进行成骨分化诱导时,发现随着碱性磷酸酶活性和羟脯氨酸的表达显著上升,在 N-乙酰半乳糖胺中含有 4,6-二硫酸盐的高硫酸化硫酸软骨素E 的含量显著升高,最终形成胶原沉积和基质矿化。高硫化硫酸软骨素E 作为信号蛋白、生长因子的配体,N-乙酰半乳糖胺中的 4,6-二硫酸盐结构为信号蛋白、生长因子提供结合位点。硫酸软骨素E 与 骨形态发生蛋白4 具有高亲和力,能与其特异性结合,进而维持稳定的信号分子浓度梯度,上调骨形态发生蛋白4通路活性,进一步增强基质矿化[36]。硫酸软骨素E 还能与钙黏附蛋白E、神经钙黏附蛋白11结合,降低 ERK1/2 的磷酸化,同时激活 SMAD3 和 SMAD1/5/8 的磷酸化,进而促进 MC3T3-E1 细胞增殖和成骨分化[37]。除了具有促进细胞成骨分化、调控骨形态发生蛋白4 的信号传递和活性等功能,硫酸软骨素及其衍生物还可增强骨髓间充质干细胞增殖能力,促使骨髓间充质干细胞从增殖型向分化型的转变,提高骨髓间充质干细胞成骨和成软骨分化能力[38-39]。由此可见,糖胺聚糖的硫酸化修饰在成骨分化调控中显得尤为重要。 与其他类型的糖胺聚糖相比,透明质酸的结构相对简单。研究表明透明质酸可以通过多种方式激活成骨信号传导通路,其具体生物学功能与分子质量以及浓度相关。高分子量透明质酸(相对分子质量2 630,2 mg/mL)显著上调成骨标志物的转录水平,有利于加快兔间充质干细胞的成骨分化,同时钙结节含量随透明质酸的分子质量和浓度升高而增多[39]。研究者们进一步探索透明质酸促进成骨分化的机制发现,高分子质量透明质酸在高浓度下通过与小鼠成肌细胞和骨髓细胞表面的 CD44 受体结合,下调了 SMAD1/5/8 的磷酸化水平,最终抑制成骨分化[40]。透明质酸还可通过下调骨形态发生蛋白 2 的拮抗剂头蛋白和卵泡抑素表达水平,抑制细胞外信号调节激酶1的磷酸化,使得 MG63 细胞中的骨形态发生蛋白2 生物活性和产出提高[41]。ZHANG 等[42]发现低分子质量透明质酸还可激活转化生长因子β/SMAD信号通路,从而诱导人羊膜间充质干的成骨分化。这提示透明质酸的分子质量和浓度可能存在交互作用从而影响其成骨作用,且不同分子质量的透明质酸影响不同阶段的细胞活动,在成骨早期阶段高分子量透明质酸在细胞外基质中迅速积累,优先促进纤维连接蛋白吸附进而对成骨细胞黏附和增殖达到正向作用。 作者总结了糖胺聚糖缺陷动物模型的骨骼系统表型信息[43-47],见表2,还归纳了糖胺聚糖在体外的成骨作用研究相关进展[48-56],见表3。"

糖胺聚糖的生物学功能之间存在一定的重叠和补偿,尽管目前的基因敲除模型研究在一定程度上揭示了其的作用,潜在的证据表明不同糖胺聚糖之间的协同效应和补偿机制使单一糖胺聚糖缺陷的模型表型可能较为轻微。大量缺乏蛋白聚糖/糖胺聚糖的动物模型表现出胚胎致死,未来应致力于建立组织特异性敲除的动物模型进一步阐明特定蛋白聚糖或糖胺聚糖链的功能。此外,研究表明糖胺聚糖影响细胞行为的方式具有细胞特异性,对成骨相关信号分子的传导至关重要,但不同的糖胺聚糖对信号通路的调控作用尚未明确。若能对不同谱系的细胞进行糖组学分析或通过系统地敲除和/或敲入蛋白多糖生物合成中编码酶的基因构建细胞文库,这将为了解特定生理环境下糖胺聚糖在不同细胞中的不同作用奠定基础,有助于更好地破译细胞系中糖胺聚糖的结构-功能关系。一方面,为了更好地破译糖胺聚糖的特定硫酸化模式,未来可通过化学酶促法合成具有明确的糖胺聚糖结构,以及利用核磁共振光谱表征技术和表面等离子共振等技术进行深入研究。 2.3 糖胺聚糖在骨组织工程中的应用 糖胺聚糖作为细胞外基质中的天然成分和信号分子,具有良好的生物相容性、可降解性、非免疫原性和可修饰性,能为细胞提供结构支持和为各种信号分子提供结合位点,因此,在组织工程的应用中具有良好的前景[57-58]。目前糖胺聚糖在骨组织工程中主要作用机制有两类:一是作为结构成分修饰细胞支架,二是作为信号调控分子参与调控成骨分化网络。 2.3.1 糖胺聚糖作为结构成分修饰细胞支架搭载细胞促进骨再生 在天然形式下,糖胺聚糖由于易降解、易溶解且缺乏良好的机械强度,使其在骨组织工程中的应用受到限制[59]。将糖胺聚糖进行化学修饰和交联,或与其他天然或合成聚合物结合形成稳定的支架,能够有效促进细胞的黏附、增殖和存活。甲基丙烯酸酯化、硫酸化和氧化是目前常见的糖胺聚糖修饰方式。糖胺聚糖上带负电荷的硫酸盐基团通过结合钙离子和磷酸盐创造了成骨适宜环境,KIM 等[60]构建了一种基于甲基丙烯酸酯化硫酸软骨素的水凝胶,他们发现硫酸软骨素水平与磷酸钙结合之间存在正相关关系,有利于原位骨的形成。另外,甲基丙烯酸化糖胺聚糖水凝胶还支持 3D 打印[61]。 生物打印可生成具有预定图案、形状和大小的定制水凝胶支架,将可光聚合的甲基丙烯酸化硫酸软骨素与热敏聚甲基丙烯酰胺-单/二乳酸酯)-聚乙二醇三嵌段共聚物结合,形成具有可调节孔隙率的结构性支架,这有利于植入的细胞保持活力和增殖[62]。除了甲基丙烯酸酯化,硫酸化是另一种常见的糖胺聚糖修饰方式,研究表明糖胺聚糖的过硫酸化可以减少炎症和促进新骨形成[63-64]。当细胞在由胶原蛋白和过硫酸化糖胺聚糖衍生物制成的人工细胞外基质上培养时,促炎因子的形成减少,抑制了细胞的炎症反应以及促进了细胞的成骨分化[65]。但基于糖胺聚糖的天然水凝胶存在机械性能不足的缺点,无法模仿天然骨组织。为了解决此局限性,学者提出可以使用合成聚合物来提高支架的机械性能。例如硫酸软骨素和超支化多功能聚乙二醇聚合物硫醇-烯反应交联形成水凝胶,不仅具有快速凝胶化、良好的机械性能和长期降解性能,还可以减少干细胞炎症反应和促进软骨修复[66]。 除了合成聚合物,糖胺聚糖还可以与天然聚合物结合,例如胶原蛋白、壳聚糖、藻酸盐和明胶等,由此进一步控制水凝胶的流变特性和机械性能。例如硫酸软骨素功能化的胶原蛋白支架可以重现软骨形成生态位、调节炎症并模仿天然胶原蛋白的机械性能[67]。不仅如此,纳米颗粒矿化胶原蛋白糖胺聚糖支架在没有外源性生长因子或干细胞的情况下,在颅骨骨缺损模型中诱导骨再生,随后研究进一步发现增加材料刚度可通过机械转导介质 YAP/TAZ 和 Wnt 信号通路诱导成骨分化[68]。最近一项研究指出基于聚乙交酯共聚物纳米纤维与透明质酸寡糖-矿化胶原蛋白微粒结合形成的细胞支架,可以介导细胞的有序排列、迁移和募集,并通过刺激细胞增殖增强骨整合[69],这说明糖胺聚糖与天然聚合物对细胞行为发挥了协同作用。 在骨修复替代材料中,糖胺聚糖的含量和浓度决定了其结构中带负电荷的硫酸盐基团与钙离子和磷酸盐的结合,从而改变支架的物理性能,为细胞创造良好的成骨环境和支持细胞黏附和增殖。通过改变藻酸盐中硫酸软骨素/锶比率(1/3,2/3 和 3/1),研究发现硫酸软骨素的硫酸盐基团增多从而能够增强亲水基团水合作用,高硫酸软骨素比率的藻酸盐水凝胶具有更好的物理性能、机械强度以及较低的弹性模量,更有利于成骨细胞黏附和增殖[70],但过量的硫酸软骨素会引起时间依赖性细胞毒性[71]。LIU 等[72]通过新型的熔融沉积成型技术制备了负载硫酸乙酰肝素的聚己内酯-羟基磷灰石 3D 多孔支架,在宏观尺度上对支架进行结构修改后表现出良好的 3D 空间结构、孔隙率、高抗压性和生物相容性。体外生物学评估发现低浓度(50 μg/mL)硫酸乙酰肝素的支架在促进成骨细胞成熟和加速骨缺损修复方面优于高浓度含(500 μg/mL)硫酸乙酰肝素支架。此外,在成骨分化初期糖胺聚糖还能优先促进纤维连接蛋白吸附至骨替代材料中,随着硫酸软骨素含量升高,吸附作用显著增强,细胞的黏附能力和生长速率明显提高。糖胺聚糖的硫酸化程度亦影响着吸附作用。低硫酸化透明质酸不仅增强了吸附作用,而且还改变了骨髓间充质干细胞纤维连接蛋白的组装状态和增加了纤维的数量,最终总纤维连接蛋白水平显著提高了两三倍 [73]。 此外,糖胺聚糖还可以和其他生物分子包括肽、环状 RGD 发挥协同作用,增强成骨作用。RACHMIEL 等[74]研发了含有透明质酸和 RGD 短肽的聚己内酯静电纺丝纤维支架,透明质酸和 RGD 短肽的加入使支架形成的纤维网状结构和形态类似于细胞外基质的纤维结构,为细胞生长和组织形成提供更为优良的仿生结构。体外实验结果表明与不含透明质酸或 RGD 短肽的支架相比,含透明质酸 和 RGD 短肽的支架组中成骨标记物的活性显著上调,缩短了间充质干细胞向成骨细胞分化的周期[74]。 在临床上,学者将糖胺聚糖应用于口腔种植修复,发现糖胺聚糖良好的成骨能力有助于加快口腔即刻种植术后种植体骨结合。REBAUDI等[75]将 96 例即刻种植牙随机分为实验组和空白对照组,实验组采用Ⅰ型胶原海绵、硫酸软骨素4 和可缓慢吸收的羟基磷灰石颗粒组成的骨替代物填充骨缺损部位,经过 4-6 个月的骨愈合期,发现实验组骨替代物加快了骨愈合和骨整合过程,形成了密度比对照组高2倍的新生骨。这些结果表明硫酸软骨素4能够增强成骨细胞的黏附与增殖能力,显著提高材料的骨诱导性能,加速即刻种植术后种植体周围骨组织的形成。在另一项前瞻性临床研究中,有研究者将透明质酸和β-磷酸三钙混合制成可注射的新型水凝胶骨替代物,该材料不仅具有足够的机械强度,还明显减少了愈合初期结缔组织的长入,其多孔结构更是为成骨细胞的长入和后续骨基质的生成沉积提供了良好的机械基础。纳入研究的21例患者在行牙拔除术后均在拔牙窝内注射该新型骨替代物,结果显示在 4 个月的愈合期后,形态学和骨计量学检查发现所有患者的牙槽窝内均有大量比正常愈合组更加致密的新骨形成,明显加快了拔牙窝愈合的时间。透明质酸的加入极大提升了该材料的生物学特性,加速内源性成骨细胞的募集,由此展示出良好的骨诱导能力,有助于加快患者拔牙窝的新骨形成,为后期的种植手术提供有利的骨量基础[76]。但目前该疗法还处于初期研究阶段,糖胺聚糖结构的异质性、不确切的作用机制和局部长期应用的安全性,使其在临床上的应用尚不普及,仍需要进一步的实验验证。 2.3.2 糖胺聚糖作为信号调控分子参与调控成骨分化网络促进骨再生 在骨组织工程中,糖胺聚糖还可作为信号调控分子为生长因子提供结合位点,保护生长因子免受蛋白酶降解,进而固定和维持稳定的信号分子浓度梯度,延长生长因子的释放时间,从而促进成骨[77]。以典型促成骨生长因子骨形态发生蛋白2 为例,有研究通过化学结合方式开发了一种胶原/骨颗粒HS3复合支架,利用糖胺聚糖模拟物 HS3与骨形态发生蛋白2 的高亲和力提高和维持支架组中骨形态发生蛋白2 的生物活性。体外缓释实验发现 HS3 复合支架降低了骨形态发生蛋白2 的初始释放速率,在27 d后支架内的骨形态发生蛋白2 含量仍有初始含量的 58%,高达对照组的3倍,显著加速了细胞的成骨分化[78]。当装载骨形态发生蛋白2 的甲基丙烯酸化硫酸软骨素支架用于治疗大鼠股骨临界骨缺损时,骨形态发生蛋白2的释放动力学得到改善,通过测量骨体积、强度和硬度发现硫酸软骨素支架诱导形成的骨量和质量与临床金标准的胶原海绵组相当[79]。另外,研究还发现调节糖胺聚糖的硫酸化程度亦能控制生长因子递送和释放。与天然硫酸软骨素相比,脱硫的硫酸软骨素在 8 d内以 1.5 倍的速率显著增加了组蛋白的释放,而硫酸化硫酸软骨素能够隔离更多的转化生长因子β1[80]。总而言之,糖胺聚糖的硫酸化模式能够有效影响生长因子的释放动力学以及影响其与细胞表面受体的结合,从而影响成骨过程[81]。 除此之外,糖胺聚糖还可以募集内源性生长因子促进生长因子与其受体结合,调控内源性生长因子的信号传导。研究者利用此作用开发了一种硫酸软骨素功能化的磷酸钙骨水泥,该材料通过聚多巴胺和二酰肼共价反应将硫酸软骨素稳定固定在其表面,利用其与重组人骨形态发生蛋白2 的非共价相互作用,将 重组人骨形态发生蛋白2有效地固定在骨水泥中,促进了重组人骨形态发生蛋白2 与其受体的结合。体外实验发现硫酸软骨素不仅促进了重组人骨形态发生蛋白2和其受体结合,同时还诱导细胞质内的受体向细胞表面移动和内源性生长因子的表达,从而上调了 p-SMAD1/5/8 和 p-ERK1/2 的磷酸化水平,形成了更成熟、更稳定的骨组织[82]。由此可见,在骨组织工程中糖胺聚糖促进内外源性生长因子与其受体结合,精确调控细胞信号传导,从而实现快速、高质量的骨再生。但是目前研究多数局限于单个糖胺聚糖在骨形成中的作用机制,参与骨形成的信号通路并不是相互孤立的,往往是协同交互式发挥作用,糖胺聚糖的生物学功能之间也存在一定的重叠和补偿,未来的研究方向是证明不同类型的糖胺聚糖以及其关键结构域在促进或抑制特定信号通路中的作用。 综上所述,糖胺聚糖经过化学修饰和交联,或与其他天然或合成聚合物联合修饰于细胞支架材料,以及作为信号分子为生长因子提供结合位点并促进内外源性生长因子与其相应受体结合,可以有效地应用于骨组织工程中实现骨再生。与基础研究相比,目前糖胺聚糖在临床上的应用尚不普及,缺乏临床中糖胺聚糖应用效果的研究。从动物组织中分离出来的糖胺聚糖因结构复杂性高和存在潜在的污染,所以其临床转化存在难度,迄今为止应用到骨组织工程中的糖胺聚糖大多数是定义不明确、异质的制剂,尽管相关的研究已充分证明其是有效的,但存在一些问题例如当使用高剂量的肝素时,肝素对生长因子存在抑制作用[83]。关于糖胺聚糖局部应用效果和安全性是未来研究的重点,可通过化学酶促法等制备糖胺聚糖库进行深入研究。另外糖胺聚糖与各信号分子的相互作用具有极强的特异性,在不同细胞和组织内,以及不同发育阶段中都存在差异,目前的研究水平还未完全破译糖胺聚糖特定的结构-功能关系,未来应充分利用糖组学全面揭示糖胺聚糖与各信号分子之间的相互协作关系,进而为解决骨再生提供更多新依据。"

| [1] YAZDANPANAH Z, JOHNSTON JD, COOPER D, et al. 3D bioprinted scaffolds for bone tissue engineering: state-of-the-art and emerging technologies. Front Bioeng Biotechnol. 2022;10:824156. [2] HAO Z, LI H, WANG Y, et al. Supramolecular peptide nanofiber hydrogels for bone tissue engineering: from multihierarchical fabrications to comprehensive applications. Adv Sci (Weinh). 2022;9(11):e2103820. [3] WU H, SHANG Y, SUN W, et al. Seamless and early gap healing of osteochondral defects by autologous mosaicplasty combined with bioactive supramolecular nanofiber-enabled gelatin methacryloyl (BSN-GelMA) hydrogel. Bioact Mater. 2023;19:88-102. [4] ALAMAN-DIEZ P, GARCIA-GARETA E, ARRUEBO M, et al. A bone-on-a-chip collagen hydrogel-based model using pre-differentiated adipose-derived stem cells for personalized bone tissue engineering. J Biomed Mater Res A. 2023;111(1):88-105. [5] GUAN Y, YANG B, XU W, et al. Cell-derived extracellular matrix materials for tissue engineering. Tissue Eng Part B Rev. 2022;28(5):1007-1021. [6] WICKRAMASINGHE ML, DIAS GJ, PREMADASA K. A novel classification of bone graft materials. J Biomed Mater Res B Appl Biomater. 2022; 110(7):1724-1749. [7] SUBBIAH R, RUEHLE MA, KLOSTERHOFF BS, et al. Triple growth factor delivery promotes functional bone regeneration following composite musculoskeletal trauma. Acta Biomater. 2021;127:180-192. [8] FANG H, ZHU D, YANG Q, et al. Emerging zero-dimensional to four-dimensional biomaterials for bone regeneration. J Nanobiotechnology. 2022;20(1):26. [9] CHEN X, CHEN D, BAN E, et al. Glycosaminoglycans modulate long-range mechanical communication between cells in collagen networks. Proc Natl Acad Sci U S A. 2022;119(15):e2116718119. [10] WU J, TIAN Y, HAN L, et al. FAM20B-catalyzed glycosaminoglycans control murine tooth number by restricting FGFR2b signaling. BMC Biol. 2020;18(1):87. [11] YU C, PEALL IW, PHAM SH, et al. Syndecan-1 facilitates the human mesenchymal stem cell osteo-adipogenic balance. Int J Mol Sci. 2020; 21(11):3884. [12] ARTIACH G, CARRACEDO M, SEIME T, et al. Proteoglycan 4 is increased in human calcified aortic valves and enhances valvular interstitial cell calcification. Cells. 2020;9(3):684. [13] HAO JX, SHEN MJ, WANG CY, et al. Regulation of biomineralization by proteoglycans: from mechanisms to application. Carbohydr Polym. 2022;294:119773. [14] GAVVA C, PATEL K, KUDRE T, et al. Glycosaminoglycans from fresh water fish processing discard -Isolation, structural characterization, and osteogenic activity. Int J Biol Macromol. 2020;145:558-567. [15] ZHENG Z, HU L, GE Y, et al. Surface modification of poly (ether ether ketone) by simple chemical grafting of strontium chondroitin sulfate to improve its anti-inflammation, angiogenesis, osteogenic properties. Adv Healthc Mater. 2022;11(13):e2200398. [16] HAYES AJ, MELROSE J. Aggrecan, the primary weight-bearing cartilage proteoglycan, has context-dependent, cell-directive properties in embryonic development and neurogenesis: aggrecan glycan side chain modifications convey interactive biodiversity. Biomolecules. 2020;10(9):1244. [17] LIMA L G, HAM S, SHIN H, et al. Tumor microenvironmental cytokines bound to cancer exosomes determine uptake by cytokine receptor-expressing cells and biodistribution. Nat Commun. 2021;12(1):3543. [18] TANG X, CHEN R, MESIAS V, et al. A SURF4-to-proteoglycan relay mechanism that mediates the sorting and secretion of a tagged variant of sonic hedgehog. Proc Natl Acad Sci U S A. 2022;119(11): e2113991119. [19] SEPURU KM, RAJARATHNAM K. Structural basis of a chemokine heterodimer binding to glycosaminoglycans. Biochem J. 2021;478(5): 1009-1021. [20] BOJARSKI KK, SAMSONOV SA. Role of oligosaccharide chain polarity in protein-glycosaminoglycan interactions. J Chem Inf Model. 2021; 61(1):455-466. [21] FU L, SUFLITA M, LINHARDT RJ. Bioengineered heparins and heparan sulfates. Adv Drug Deliv Rev. 2016;97:237-249. [22] VARKI A, CUMMINGS RD, AEBI M, et al. Symbol nomenclature for graphical representations of glycans. Glycobiology. 2015;25(12):1323-1324. [23] MIGLIORINI E, GUEVARA-GARCIA A, ALBIGES-RIZO C, et al. Learning from BMPs and their biophysical extracellular matrix microenvironment for biomaterial design. Bone. 2020;141:115540. [24] SOARES DCD, REIS RL, PASHKULEVA I. Sulfation of glycosaminoglycans and its implications in human health and disorders. Annu Rev Biomed Eng. 2017;19:1-26. [25] IOZZO RV, SCHAEFER L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42: 11-55. [26] VLODAVSKY I, BARASH U, NGUYEN HM, et al. Biology of the heparanase-heparan sulfate axis and its role in disease pathogenesis. Semin Thromb Hemost. 2021;47(3):240-253. [27] FUNDERBURGH JL. Keratan sulfate: structure, biosynthesis, and function. Glycobiology. 2000;10(10):951-958. [28] IDA-YONEMOCHI H, MORITA W, SUGIURA N, et al. Craniofacial abnormality with skeletal dysplasia in mice lacking chondroitin sulfate N-acetylgalactosaminyltransferase-1. Sci Rep. 2018;8(1):17134. [29] SHABBIR R, NALBANT G, AHMAD N, et al. Homozygous CHST11 mutation in chondrodysplasia, brachydactyly, overriding digits, clino-symphalangism and synpolydactyly. J Med Genet. 2018;55(7):489-496. [30] SCHNEEBERGER PE, VON ELSNER L, BARKER EL, et al. Bi-allelic pathogenic variants in hs2st1 cause a syndrome characterized by developmental delay and corpus callosum, skeletal, and renal abnormalities. Am J Hum Genet. 2020;107(6):1044-1061. [31] NURCOMBE V, GOH FJ, HAUPT LM, et al. Temporal and functional changes in glycosaminoglycan expression during osteogenesis. J Mol Histol. 2007; 38(5):469-481. [32] GRIFFIN ME, SORUM AW, MILLER GM, et al. Sulfated glycans engage the Ang-Tie pathway to regulate vascular development. Nat Chem Biol. 2021;17(2):178-186. [33] MURALI S, RAI B, DOMBROWSKI C, et al. Affinity-selected heparan sulfate for bone repair. Biomaterials. 2013;34(22):5594-5605. [34] SMITH R, MURALI S, RAI B, et al. Minimum structural requirements for BMP-2-binding of heparin oligosaccharides. Biomaterials. 2018;184: 41-55. [35] HUA R, NI Q, ELIASON T D, et al. Biglycan and chondroitin sulfate play pivotal roles in bone toughness via retaining bound water in bone mineral matrix. Matrix Biol. 2020;94:95-109. [36] MIYAZAKI T, MIYAUCHI S, TAWADA A, et al. Oversulfated chondroitin sulfate-E binds to BMP-4 and enhances osteoblast differentiation. J Cell Physiol. 2008;217(3):769-777. [37] KOIKE T, IZUMIKAWA T, TAMURA J, et al. Chondroitin sulfate-E fine-tunes osteoblast differentiation via ERK1/2, Smad3 and Smad1/5/8 signaling by binding to N-cadherin and cadherin-11. Biochem Biophys Res Commun. 2012;420(3):523-529. [38] SHANG L, MA B, WANG F, et al. Nanotextured silk fibroin/hydroxyapatite biomimetic bilayer tough structure regulated osteogenic/chondrogenic differentiation of mesenchymal stem cells for osteochondral repair. Cell Prolif. 2020;53(11):e12917. [39] LI X, TENG Y, LIU J, et al. Chondrogenic differentiation of BMSCs encapsulated in chondroinductive polysaccharide/collagen hybrid hydrogels. J Mater Chem B. 2017;5(26):5109-5119. [40] KANEKO K, HIGUCHI C, KUNUGIZA Y, et al. Hyaluronan inhibits BMP-induced osteoblast differentiation. FEBS Lett. 2015;589(4):447-454. [41] KAWANO M, ARIYOSHI W, IWANAGA K, et al. Mechanism involved in enhancement of osteoblast differentiation by hyaluronic acid. Biochem Biophys Res Commun. 2011;405(4):575-580. [42] ZHANG LT, LIU RM, LUO Y, et al. Hyaluronic acid promotes osteogenic differentiation of human amniotic mesenchymal stem cells via the TGF-beta/Smad signalling pathway. Life Sci. 2019;232:116669. [43] CORSI A, XU T, CHEN XD, et al. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J Bone Miner Res. 2002;17(7):1180-1189. [44] MATSUMOTO Y, MATSUMOTO K, IRIE F, et al. Conditional ablation of the heparan sulfate-synthesizing enzyme Ext1 leads to dysregulation of bone morphogenic protein signaling and severe skeletal defects. J Biol Chem. 2010;285(25):19227-19234. [45] HAYASHI M, KADOMATSU K, KOJIMA T, et al. Keratan sulfate and related murine glycosylation can suppress murine cartilage damage in vitro and in vivo. Biochem Biophys Res Commun. 2011;409(4):732-737. [46] CHAN WL, STEINER M, WITKOS T, et al. Impaired proteoglycan glycosylation, elevated TGF-beta signaling, and abnormal osteoblast differentiation as the basis for bone fragility in a mouse model for gerodermia osteodysplastica. PLoS Genet. 2018;14(3):e1007242. [47] KAWASHIMA K, OGAWA H, KOMURA S, et al. Heparan sulfate deficiency leads to hypertrophic chondrocytes by increasing bone morphogenetic protein signaling. Osteoarthritis Cartilage. 2020;28(11):1459-1470. [48] HAUPT LM, MURALI S, MUN FK, et al. The heparan sulfate proteoglycan (HSPG) glypican-3 mediates commitment of MC3T3-E1 cells toward osteogenesis. J Cell Physiol. 2009;220(3):780-791. [49] LING L, DOMBROWSKI C, FOONG KM, et al. Synergism between Wnt3a and heparin enhances osteogenesis via a phosphoinositide 3-kinase/Akt/RUNX2 pathway. J Biol Chem. 2010;285(34):26233-26244. [50] BRAMONO DS, MURALI S, RAI B, et al. Bone marrow-derived heparan sulfate potentiates the osteogenic activity of bone morphogenetic protein-2 (BMP-2). Bone. 2012;50(4):954-964. [51] WU B, MA X, ZHU D, et al. Lentiviral delivery of biglycan promotes proliferation and increases osteogenic potential of bone marrow-derived mesenchymal stem cells in vitro. J Mol Histol. 2013;44(4):423-431. [52] SALBACH-HIRSCH J, ZIEGLER N, THIELE S, et al. Sulfated glycosaminoglycans support osteoblast functions and concurrently suppress osteoclasts. J Cell Biochem. 2014;115(6):1101-1111. [53] MATHEWS S, MATHEW SA, GUPTA PK, et al. Glycosaminoglycans enhance osteoblast differentiation of bone marrow derived human mesenchymal stem cells. J Tissue Eng Regen Med. 2014;8(2):143-152. [54] SIMANN M, SCHNEIDER V, LE BLANC S, et al. Heparin affects human bone marrow stromal cell fate: promoting osteogenic and reducing adipogenic differentiation and conversion. Bone. 2015;78:102-113. [55] LIN W, ZHU X, GAO L, et al. Osteomodulin positively regulates osteogenesis through interaction with BMP2. Cell Death Dis. 2021; 12(2):147. [56] OTSUKA T, KAN HM, MASON TD, et al. Overexpression of NDST1 attenuates fibrotic response in murine adipose-derived stem cells. Stem Cells Dev. 2022. doi: 10.1089/scd.2022.0053. [57] PARK SS, PARK M, LEE BT. Autologous stromal vascular fraction-loaded hyaluronic acid/gelatin-biphasic calcium phosphate scaffold for bone tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2022;132:112533. [58] KLARA J, MARCZAK A, LATKIEWICZ A, et al. Lysine-functionalized chondroitin sulfate improves the biological properties of collagen/chitosan-based injectable hydrogels. Int J Biol Macromol. 2022;202: 318-331. [59] D’ALBIS G, D’ALBIS V, PALMA M, et al. Use of hyaluronic acid for regeneration of maxillofacial bones. Genesis. 2022;60(8-9):e23497. [60] KIM HD, LEE EA, AN YH, et al. Chondroitin sulfate-based biomineralizing surface hydrogels for bone tissue engineering. ACS Appl Mater Interfaces. 2017;9(26):21639-21650. [61] POLDERVAART MT, GOVERSEN B, DE RUIJTER M, et al. 3D bioprinting of methacrylated hyaluronic acid (MeHA) hydrogel with intrinsic osteogenicity. PLoS One. 2017;12(6):e177628. [62] ABBADESSA A, BLOKZIJL MM, MOUSER VH, et al. A thermo-responsive and photo-polymerizable chondroitin sulfate-based hydrogel for 3D printing applications. Carbohydr Polym. 2016;149:163-174. [63] KRIEGHOFF J, PICKE AK, SALBACH-HIRSCH J, et al. Increased pore size of scaffolds improves coating efficiency with sulfated hyaluronan and mineralization capacity of osteoblasts. Biomater Res. 2019;23:26. [64] HAUCK S, ZAGER P, HALFTER N, et al. Collagen/hyaluronan based hydrogels releasing sulfated hyaluronan improve dermal wound healing in diabetic mice via reducing inflammatory macrophage activity. Bioact Mater. 2021;6(12):4342-4359. [65] HEMPEL U, MATTHAUS C, PREISSLER C, et al. Artificial matrices with high-sulfated glycosaminoglycans and collagen are anti-inflammatory and pro-osteogenic for human mesenchymal stromal cells. J Cell Biochem. 2014;115(9):1561-1571. [66] LI X, XU Q, JOHNSON M, et al. A chondroitin sulfate based injectable hydrogel for delivery of stem cells in cartilage regeneration. Biomater Sci. 2021;9(11):4139-4148. [67] CORRADETTI B, TARABALLI F, MINARDI S, et al. Chondroitin sulfate immobilized on a biomimetic scaffold modulates inflammation while driving chondrogenesis. Stem Cells Transl Med. 2016;5(5):670-682. [68] ZHOU Q, LYU S, BERTRAND AA, et al. Stiffness of nanoparticulate mineralized collagen scaffolds triggers osteogenesis via mechanotransduction and canonical wnt signaling. Macromol Biosci. 2021;21(3):e2000370. [69] LIU L, JIA W, ZHOU Y, et al. Hyaluronic acid oligosaccharide-collagen mineralized product and aligned nanofibers with enhanced vascularization properties in bone tissue engineering. Int J Biol Macromol. 2022;206:277-287. [70] FENBO M, SIJING L, RUIZ-ORTEGA LI, et al. Effects of alginate/chondroitin sulfate-based hydrogels on bone defects healing. Mater Sci Eng C Mater Biol Appl. 2020;116:111217. [71] KIM YS, GUO JL, LAM J, et al. Synthesis of injectable, thermally responsive, chondroitin sulfate-cross-linked Poly (N-isopropylacrylamide) hydrogels. ACS Biomater Sci Eng. 2019;5(12):6405-6413. [72] LIU Y, WANG R, CHEN S, et al. Heparan sulfate loaded polycaprolactone-hydroxyapatite scaffolds with 3D printing for bone defect repair. Int J Biol Macromol. 2020;148:153-162. [73] SHI H, YE X, ZHANG J, et al. Enhanced osteogenesis of injectable calcium phosphate bone cement mediated by loading chondroitin sulfate. ACS Biomater Sci Eng. 2019;5(1):262-271. [74] RACHMIEL D, ANCONINA I, RUDNICK-GLICK S, et al. Hyaluronic acid and a short peptide improve the performance of a pcl electrospun fibrous scaffold designed for bone tissue engineering applications. Int J Mol Sci. 2021;22(5):2425. [75] REBAUDI A, SILVESTRINI P, TRISI P. Use of a resorbable hydroxyapatite-collagen chondroitin sulfate material on immediate postextraction sites: a clinical and histologic study. Int J Periodontics Restorative Dent. 2003;23(4):371-379. [76] LORENZ J, BARBECK M, KIRKPATRICK CJ, et al. Injectable bone substitute material on the basis of beta-tcp and hyaluronan achieves complete bone regeneration while undergoing nearly complete degradation. Int J Oral Maxillofac Implants. 2018;33(3):636-644. [77] GAO X, HWANG M P, WRIGHT N, et al. The use of heparin/polycation coacervate sustain release system to compare the bone regenerative potentials of 5 BMPs using a critical sized calvarial bone defect model. Biomaterials. 2022;288:121708. [78] QUANG LB, CHUN TT, LEE SB, et al. A biomimetic collagen-bone granule-heparan sulfate combination scaffold for BMP2 delivery. Gene. 2021;769:145217. [79] ANDREWS S, CHENG A, STEVENS H, et al. Chondroitin sulfate glycosaminoglycan scaffolds for cell and recombinant protein-based bone regeneration. Stem Cells Transl Med. 2019;8(6):575-585. [80] LIM JJ, TEMENOFF JS. The effect of desulfation of chondroitin sulfate on interactions with positively charged growth factors and upregulation of cartilaginous markers in encapsulated MSCs. Biomaterials. 2013; 34(21):5007-5018. [81] KARUMBAIAH L, ENAM SF, BROWN AC, et al. Chondroitin sulfate glycosaminoglycan hydrogels create endogenous niches for neural stem cells. Bioconjug Chem. 2015;26(12):2336-2349. [82] HUANG B, WU Z, DING S, et al. Localization and promotion of recombinant human bone morphogenetic protein-2 bioactivity on extracellular matrix mimetic chondroitin sulfate-functionalized calcium phosphate cement scaffolds. Acta Biomater. 2018;71:184-199. [83] LI Y, LIU L, LI S, et al. Impaired bone healing by enoxaparin via inhibiting the differentiation of bone marrow mesenchymal stem cells towards osteoblasts. J Bone Miner Metab. 2022;40(1):9-19. [84] CHEN YH, NARIMATSU Y, CLAUSEN TM, et al. The GAGOme: a cell-based library of displayed glycosaminoglycans. Nat Methods. 2018; 15(11):881-888. [85] POMIN VH, WANG X. Synthetic oligosaccharide libraries and microarray technology: a powerful combination for the success of current glycosaminoglycan interactomics. Chem Med Chem. 2018;13(7):648-661. |
| [1] | Dang Yi, Du Chengyan, Yao Honglin, Yuan Nenghua, Cao Jin, Xiong Shan, Zhang Dingmei, Wang Xin. Hormonal osteonecrosis and oxidative stress [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1469-1476. |
| [2] | Yang Zhishan, Tang Zhenglong. YAP/TAZ, a core factor of the Hippo signaling pathway, is involved in bone formation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1264-1271. |
| [3] | Liu Xiaolin, Mu Xinyue, Ma Ziyu, Liu Shutai, Wang Wenlong, Han Xiaoqian, Dong Zhiheng. Effect of hydrogel-loaded simvastatin microspheres on osteoblast proliferation and differentiation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 998-1003. |
| [4] | Tang Haotian, Liao Rongdong, Tian Jing. Application and design of piezoelectric materials for bone defect repair [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1117-1125. |
| [5] | Xu Yan, Li Ping, Lai Chunhua, Zhu Peijun, Yang Shuo, Xu Shulan. Piezoelectric materials for vascularized bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1126-1132. |
| [6] | Liu Wentao, Feng Xingchao, Yang Yi, Bai Shengbin. Effect of M2 macrophage-derived exosomes on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 840-845. |
| [7] | Long Yanming, Xie Mengsheng, Huang Jiajie, Xue Wenli, Rong Hui, Li Xiaojie. Casein kinase 2-interaction protein-1 regulates the osteogenic ability of bone marrow mesenchymal stem cells in osteoporosis rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 878-882. |
| [8] | Li Xinyue, Li Xiheng, Mao Tianjiao, Tang Liang, Li Jiang. Three-dimensional culture affects morphology, activity and osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 846-852. |
| [9] | Yuan Wei, Liu Jingdong, Xu Guanghui, Kang Jian, Li Fuping, Wang Yingjie, Zhi Zhongzheng, Li Guanwu. Osteogenic differentiation of human perivascular stem cells and its regulation based on Wnt/beta-catenin signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 866-871. |
| [10] | Qiao Luhui, Ma Ziyu, Guo Haoyu, Hou Yudong. Comparison of puerarin and icariin on the biological properties of mouse preosteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 872-877. |
| [11] | Qin Yuxing, Ren Qiangui, Li Zilong, Quan Jiaxing, Shen Peifeng, Sun Tao, Wang Haoyu. Action mechanism and prospect of bone microvascular endothelial cells for treating femoral head necrosis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 955-961. |
| [12] | Zhang Min, Zhang Xiaoming, Liu Tongbin. Application potential of naringin in bone tissue regeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 787-792. |
| [13] | Huang Qian, Hao Liying, He Longlong, Du Liangzhi. Graphene oxide-chitosan composite coating affects the biological behavior of osteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5430-5435. |
| [14] | Liu Zixuan, Li Yan, Ji Lin, Xia Delin. Biological properties of nano-hydroxyapatite-zinc oxide composite scaffolds and their effects on the behavior of MC3T3-E1 osteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5441-5447. |
| [15] | Li Li, Li Xiao, Li Duchenhui, Zhang Jie, Xiao Tianjiao, Kang Jiabing, Tian Ai. Regulation of interleukin-4 on osteoclast differentiation during bone regeneration guided by bone replacement materials [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5455-5461. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||