Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (6): 866-871.doi: 10.12307/2023.250
Previous Articles Next Articles
Osteogenic differentiation of human perivascular stem cells and its regulation based on Wnt/beta-catenin signaling pathway
Yuan Wei1, Liu Jingdong1, Xu Guanghui1, Kang Jian1, Li Fuping1, Wang Yingjie1, Zhi Zhongzheng1, Li Guanwu2
- 1Department of Spine Surgery, Shanghai Fourth People’s Hospital, Tongji University, Shanghai 200434, China; 2Department of Radiology, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai 200437, China
-
Received:2022-02-08Accepted:2022-04-23Online:2023-02-28Published:2022-08-11 -
Contact:Liu Jingdong, Chief physician, Department of Spine Surgery, Shanghai Fourth People’s Hospital, Tongji University, Shanghai 200434, China Li Guanwu, MD, Associate chief physician, Department of Radiology, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai 200437, China -
About author:Yuan Wei, MD, Department of Spine Surgery, Shanghai Fourth People’s Hospital, Tongji University, Shanghai 200434, China -
Supported by:the General Program of Shanghai Municipal Health Commission, No. 202040317 (to YW); Academic Subject Boosting Plan in the Shanghai Fourth People’s Hospital, No. SY-XKZT-2021-1009 (to YW); Key Support to Specialty-Funded Project of Shanghai Hongkou District, No. HKZK2020A07 (to XGH)
CLC Number:
Cite this article
Yuan Wei, Liu Jingdong, Xu Guanghui, Kang Jian, Li Fuping, Wang Yingjie, Zhi Zhongzheng, Li Guanwu. Osteogenic differentiation of human perivascular stem cells and its regulation based on Wnt/beta-catenin signaling pathway[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 866-871.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
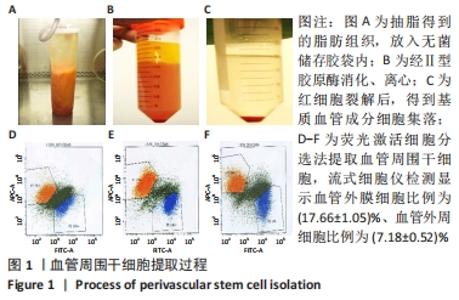
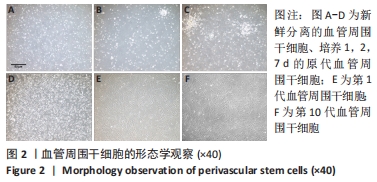
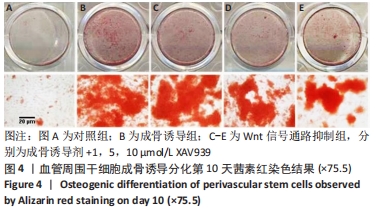
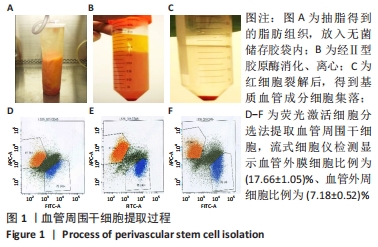
2.1 血管周围干细胞特点分析 2.1.1 基质血管成分细胞的比例 脂肪组织通过Ⅱ型胶原酶消化、离心,得到基质血管成分细胞,见图1A-C。每100 mL脂肪中平均含有总的基质血管成分细胞为(1.82±0.32)×107,通过锥虫蓝拒染法检测细胞活性,活细胞数目占(82.72±5.37)%。 2.1.2 血管周围干细胞比例 基质血管成分细胞由多种细胞群组成,除了脂肪干细胞以外,还包括血细胞、纤维母细胞和内皮细胞,也有前体脂肪细胞或原始脂肪细胞,它是一种杂合体。通过荧光激活细胞分选法筛选出CD34+、CD45-、CD146-和CD146+、CD45-、CD34-两类细胞,把它们合并称为血管周围干细胞。进一步分析显示,血管外膜细胞(CD34+、CD45-、CD146-)比例为(17.66±1.05)%;血管外周细胞(CD146+、CD45-、CD34-)比例为(7.18±0.52)%,见图1D-F。"
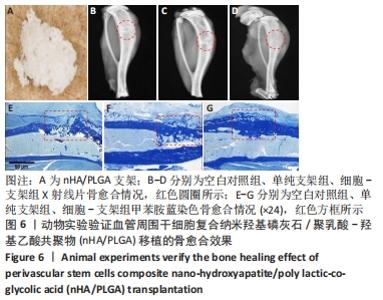
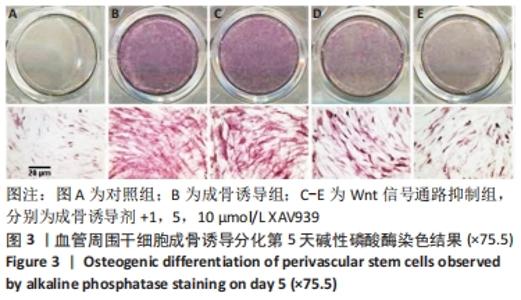
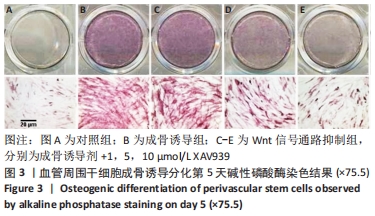
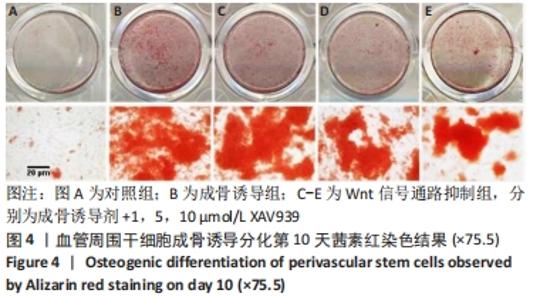
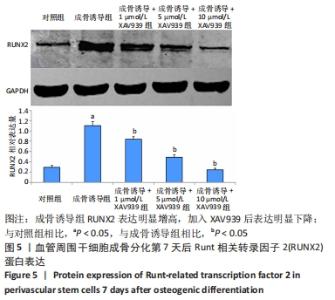
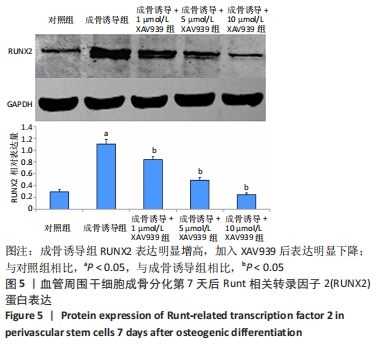
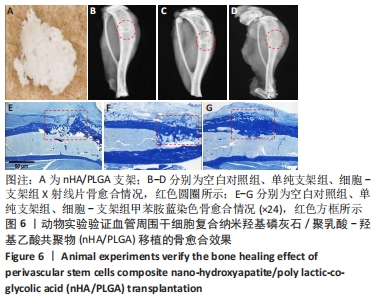
2.5 实验动物骨缺损愈合情况 18只小鼠均进入结果分析。血管周围干细胞与nHA/PLGA支架复合后,植入胫骨骨缺损处,见图6A。术后14 d,X射线片显示细胞-支架组骨缺损区已被大量骨组织取代、皮质骨厚度明显增加,与周围骨组织密度一致,骨形态上与正常骨组织无明显区别;单纯支架组虽有骨痂形成,但骨缺损区存在低密度影,皮质骨密度低于周围正常骨组织;未植入任何材料,即空白对照组显示骨缺损区较清晰,有明显透亮影,见图6B-D红色圆圈所示。 甲苯胺蓝染色显示细胞-支架组骨缺损区大量新生骨痂已转化为正常骨组织,支架材料已被降解吸收;单纯支架组骨缺损区形成的骨组织相对较少,可见增生的软骨基质;空白对照组骨缺损区见大量纤维组织填充,仅有少量骨组织,见图6E-G红色方框所示。 "
| [1] GALIPEAU J, SENSÉBÉ L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell. 2018;22(6):824-833. [2] VASANTHAN J, GURUSAMY N, RAJASINGH S, et al. Role of Human Mesenchymal Stem Cells in Regenerative Therapy. Cells. 2020;10(1):54. [3] RODRÍGUEZ-FUENTES DE, FERNÁNDEZ-GARZA LE, SAMIA-MEZA JA, et al. Mesenchymal Stem Cells Current Clinical Applications: A Systematic Review. Arch Med Res. 2021;52(1):93-101. [4] SCHÄFFLER A, BÜCHLER C. Concise review: adipose tissue-derived stromal cells--basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25(4):818-827. [5] RAPOSIO E, BONOMINI S, CALDERAZZI F. Isolation of autologous adipose tissue-derived mesenchymal stem cells for bone repair. Orthop Traumatol Surg Res. 2016;102(7):909-912. [6] MURATA D, FUJIMOTO R, NAKAYAMA K. Osteochondral Regeneration Using Adipose Tissue-Derived Mesenchymal Stem Cells. Int J Mol Sci. 2020;21(10):3589. [7] CRISAN M, YAP S, CASTEILLA L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301-313. [8] JAMES AW, ZARA JN, CORSELLI M, et al. Use of human perivascular stem cells for bone regeneration. J Vis Exp. 2012;63:e2952. [9] XU W, SUN Y, ZHANG J, et al. Perivascular-derived stem cells with neural crest characteristics are involved in tendon repair. Stem Cells Dev. 2015; 24(7):857-868. [10] SBIERSKI-KIND J, MROZ N, MOLOFSKY AB. Perivascular stromal cells: Directors of tissue immune niches. Immunol Rev. 2021;302(1):10-31. [11] YUAN Z, LI Q, LUO S, et al. PPARgamma and Wnt Signaling in Adipogenic and Osteogenic Differentiation of Mesenchymal Stem Cells. Curr Stem Cell Res Ther. 2016;11(3):216-225. [12] HAN H, TIAN T, HUANG G, et al. The lncRNA H19/miR-541-3p/Wnt/beta-catenin axis plays a vital role in melatonin-mediated osteogenic differentiation of bone marrow mesenchymal stem cells. Aging (Albany NY). 2021;13(14):18257-18273. [13] YANG F, YANG D, TU J, et al. Strontium enhances osteogenic differentiation of mesenchymal stem cells and in vivo bone formation by activating Wnt/catenin signaling. Stem Cells. 2011;29(6):981-991. [14] CORSELLI M, CRISAN M, MURRAY IR, et al. Identification of perivascular mesenchymal stromal/stem cells by flow cytometry. Cytometry A. 2013;83(8):714-720. [15] CUI Z, LI C, JIANG N, et al. Isolation and characterization of minipig perivascular stem cells for bone tissue engineering. Mol Med Rep. 2018;18(4):3555-3562. [16] LIANG CY, LUO YC, YANG GD, et al .Graphene Oxide Hybridized nHAC/PLGA Scaffolds Facilitate the Proliferation of MC3T3-E1 Cells. Nanoscale Res Lett. 2018;13(1):15. [17] YAN J, LIU C, TU C, et al. Hydrogel-hydroxyapatite-monomeric collagen type-I scaffold with low-frequency electromagnetic field treatment enhances osteochondral repair in rabbits. Stem Cell Res Ther. 2021; 12(1):572. [18] KIM JM, HONG KS, SONG WK, et al. Perivascular Progenitor Cells Derived From Human Embryonic Stem Cells Exhibit Functional Characteristics of Pericytes and Improve the Retinal Vasculature in a Rodent Model of Diabetic Retinopathy. Stem Cells Transl Med. 2016; 5(9):1268-1276. [19] XU J, WANG Y, HSU CY, et al. Human perivascular stem cell-derived extracellular vesicles mediate bone repair. Elife. 2019;8:e48191. [20] JAMES AW, PÉAULT B. Perivascular Mesenchymal Progenitors for Bone Regeneration. J Orthop Res. 2019;37(6):1221-1228. [21] CORSELLI M, CHEN CW, CRISAN M, et al. Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol. 2010;30(6): 1104-1109. [22] CANO E, GEBALA V, GERHARDT H. Pericytes or Mesenchymal Stem Cells: Is That the Question? Cell Stem Cell. 2017;20(3):296-297. [23] RIBEIRO AJ, TOTTEY S, TAYLOR RW, et al. Mechanical characterization of adult stem cells from bone marrow and perivascular niches. J Biomech. 2012;45(7):1280-1287. [24] LI CS, ZHANG X, PÉAULT B, et al. Accelerated Chondrogenic Differentiation of Human Perivascular Stem Cells with NELL-1. Tissue Eng Part A. 2016;22(3-4):272-285. [25] XU J, WANG Y, GOMEZ-SALAZAR MA, et al. Bone-forming perivascular cells: Cellular heterogeneity and use for tissue repair. Stem Cells. 2021; 39(11):1427-1434. [26] THELEN K, AYALA-LOPEZ N, WATTS SW, et al. Expansion and Adipogenesis Induction of Adipocyte Progenitors from Perivascular Adipose Tissue Isolated by Magnetic Activated Cell Sorting. J Vis Exp. 2017;(124):55818. [27] MEYERS CA, XU J, ZHANG L, et al. Skeletogenic Capacity of Human Perivascular Stem Cells Obtained Via Magnetic-Activated Cell Sorting. Tissue Eng Part A. 2019;25(23-24):1658-1666. [28] DENG Q, LI P, CHE M, et al. Activation of hedgehog signaling in mesenchymal stem cells induces cartilage and bone tumor formation via Wnt/beta-Catenin. Elife. 2019;8:e50208. [29] WANG Y, ZHANG X, SHAO J, et al. Adiponectin regulates BMSC osteogenic differentiation and osteogenesis through the Wnt/beta-catenin pathway. Sci Rep. 2017;7(1):3652. [30] HUANG W, ZHENG X, YANG X, et al. Stimulation of Osteogenic Differentiation by Saikosaponin-A in Bone Marrow Stromal Cells Via WNT/β-Catenin Pathway. Calcif Tissue Int. 2017;100(4):392-401. [31] ALMASOUD N, BINHAMDAN S, YOUNIS G, et al. Tankyrase inhibitor XAV-939 enhances osteoblastogenesis and mineralization of human skeletal (mesenchymal) stem cells. Sci Rep. 2020;10(1):16746. [32] LI M, ZHANG C, LI X, et al. Isoquercitrin promotes the osteogenic differentiation of osteoblasts and BMSCs via the RUNX2 or BMP pathway. Connect Tissue Res. 2019;60(2):189-199. [33] CAI T, SUN D, DUAN Y, et al. WNT/beta-catenin signaling promotes VSMCs to osteogenic transdifferentiation and calcification through directly modulating Runx2 gene expression. Exp Cell Res. 2016;345(2): 206-217. [34] SHEN J, CHEN X, JIA H, et al. Effects of WNT3A and WNT16 on the Osteogenic and Adipogenic Differentiation of Perivascular Stem/Stromal Cells. Tissue Eng Part A. 2018;24(1-2):68-80. [35] BACAKOVA L, ZARUBOVA J, TRAVNICKOVA M, et al. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells - a review. Biotechnol Adv. 2018;36(4): 1111-1126. |
| [1] | Zhao Lu, Zhao Yifei, Gao Da, Liu Yanfang, Fu Tingting, Xu Jiangyan. Expression of suppressor of Zeste 12 in kidney tissues of rats with diabetic nephropathy [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1179-1186. |
| [2] | Yang Zhishan, Tang Zhenglong. YAP/TAZ, a core factor of the Hippo signaling pathway, is involved in bone formation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1264-1271. |
| [3] | Yuan Bo, Xie Lide, Fu Xiumei. Schwann cell-derived exosomes promote the repair and regeneration of injured peripheral nerves [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 935-940. |
| [4] | Liu Wentao, Feng Xingchao, Yang Yi, Bai Shengbin. Effect of M2 macrophage-derived exosomes on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 840-845. |
| [5] | Long Yanming, Xie Mengsheng, Huang Jiajie, Xue Wenli, Rong Hui, Li Xiaojie. Casein kinase 2-interaction protein-1 regulates the osteogenic ability of bone marrow mesenchymal stem cells in osteoporosis rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 878-882. |
| [6] | Li Xinyue, Li Xiheng, Mao Tianjiao, Tang Liang, Li Jiang. Three-dimensional culture affects morphology, activity and osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 846-852. |
| [7] | Wang Jinling, Huang Xiarong, Qu Mengjian, Huang Fujin, Yin Lingwei, Zhong Peirui, Liu Jin, Sun Guanghua, Liao Yang, Zhou Jun. Effects of exercise training on bone mass and bone microstructure in aged osteoporotic rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 676-682. |
| [8] | Wu Yujie, Wan Xiaofang, Wei Mianxing, Peng Shiyuan, Xu Xiaomei. Correlation between autophagy and the Hippo-YAP protein pathway in periodental ligament cells on the pressure side of a mouse model of orthodontic tooth movement [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 683-689. |
| [9] | Tao Xin, Xu Yi, Song Zhiwen, Liu Jinbo. Hippo signaling pathway in the regulation of spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(4): 619-625. |
| [10] | Dai Xianglin, Zhang Wenfeng, Yao Xijun, Shang Jiaqi, Huang Qiujin, Ren Yifan, Deng Jiupeng. Barium titanate/polylactic acid piezoelectric composite film affects adhesion, proliferation, and osteogenic differentiation of MC3T3-E1 cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 367-373. |
| [11] | Li Rui, Liu Zhen, Guo Zige, Lu Ruijie, Wang Chen. Aspirin-loaded chitosan nanoparticles and polydopamine modified titanium sheets improve osteogenic differentiation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 374-379. |
| [12] | Luo Di, Liang Xuezhen, Yan Bozhao, Li Jiacheng, Xu Bo, Li Gang. Mechanism of Bushen Huoxue Capsule in repair of bone defects due to steroid-induced osteonecrosis of the femoral head in rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(2): 184-191. |
| [13] | Zhao Feng, Fan Shaoqing, Cheng Xiaoyan, Li Xiaona, Li Changsheng, Ma Haojie. miR-132-3p targets and modulates Ptch1 to reduce neuropathic pain in mice with chronic constriction injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(2): 230-236. |
| [14] | Zhong Jinglin, Cao Huimin, Pan Yaru, Jian Wenxuan, Wang Qi. Ginsenoside Rg3 protects PC12 cells against oxygen-glucose deprivation/reoxygenation-induced damage [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(2): 177-183. |
| [15] | Zhang Jie, Tian Ai. Advances in the signaling pathway of M2 macrophages involved in bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(2): 314-321. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||