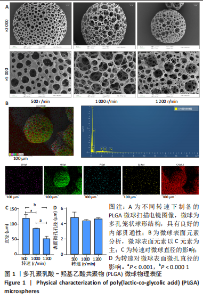
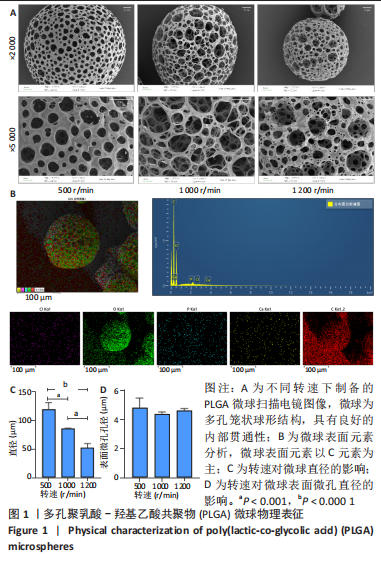
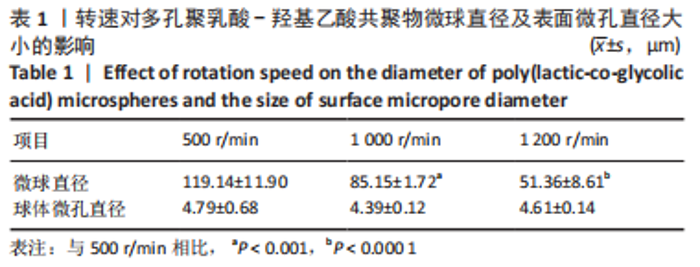
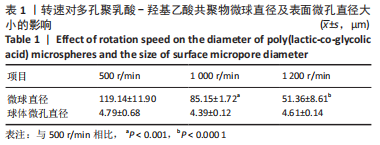
Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (34): 5469-5476.doi: 10.12307/2023.557
Previous Articles Next Articles
Construction and in vivo osteogenesis of microspheres loaded with immunomodulatory peptide/miR-26a complexes for slow release
Li Xinlun1, Zhu Yushu1, Yang Yiling2, He Siqi3, Wen Nan3, Mu Yandong1, 3
- 1School of Stomatology, Southwest Medical University, Luzhou 646000, Sichuan Province, China; 2North Sichuan Medical College, Nanchong 637000, Sichuan Province, China; 3Sichuan Academy of Medical Sciences · Sichuan Provincial People’s Hospital, Chengdu 610072, Sichuan Province, China
-
Received:2022-09-13Accepted:2022-10-28Online:2023-12-08Published:2023-04-20 -
Contact:Mu Yandong, Chief physician, School of Stomatology, Southwest Medical University, Luzhou 646000, Sichuan Province, China; Sichuan Academy of Medical Sciences · Sichuan Provincial People’s Hospital, Chengdu 610072, Sichuan Province, China -
About author:Li Xinlun, Master candidate, Physician, School of Stomatology, Southwest Medical University, Luzhou 646000, Sichuan Province, China -
Supported by:General Program of National Natural Science Foundation of China, No. 82071168 (to MYD); Key Research & Development Project of Sichuan Provincial Department of Science and Technology, No. 2021YFS0009 (to MYD)
CLC Number:
Cite this article
Li Xinlun, Zhu Yushu, Yang Yiling, He Siqi, Wen Nan, Mu Yandong. Construction and in vivo osteogenesis of microspheres loaded with immunomodulatory peptide/miR-26a complexes for slow release[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5469-5476.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
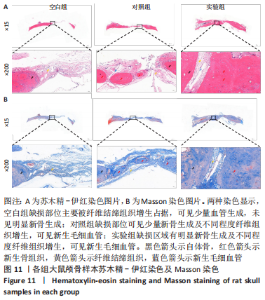
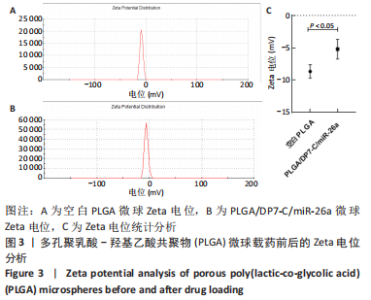
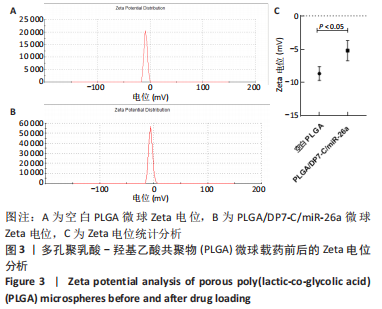
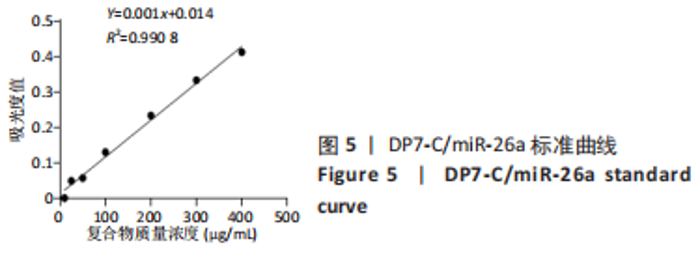
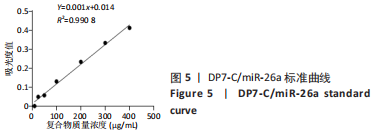
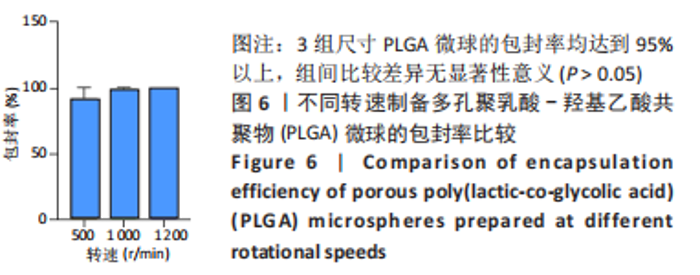
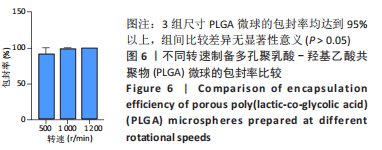
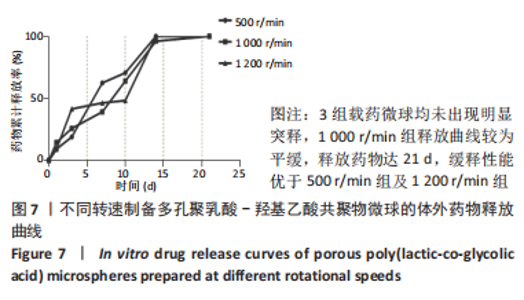
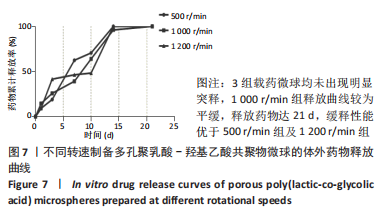
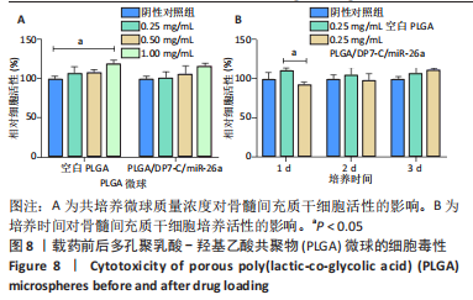
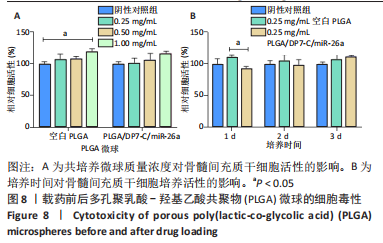
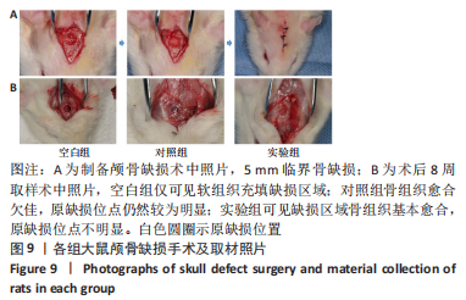
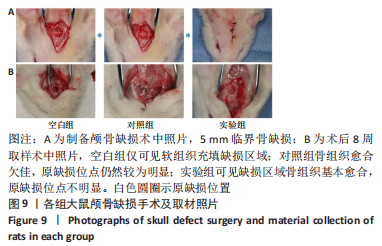
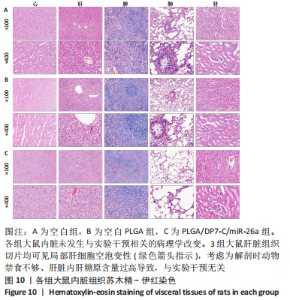
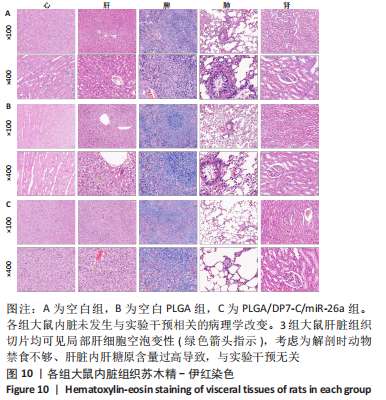
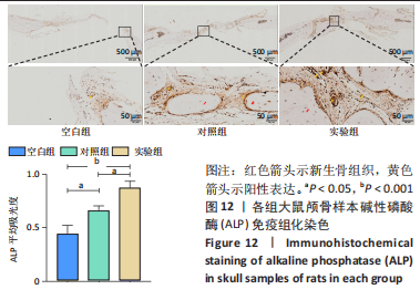
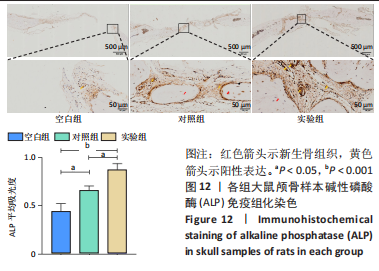
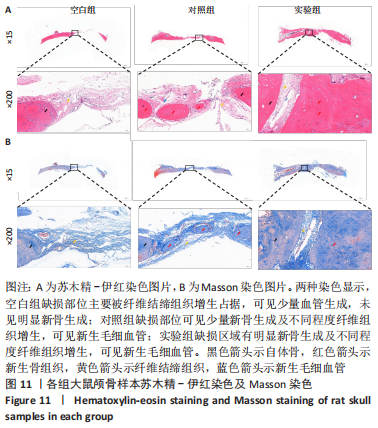
各组大鼠心脏组织结构完整,未见明显增生或炎性浸润,间质未见明显炎性细胞浸润和纤维组织增生。各组大鼠肝脏组织结构及被膜完整、清晰,肝窦未见淤血扩张,局部可见肝细胞空泡变性。各组大鼠脾脏组织被膜完整,被膜及脾小梁纤维结缔组织未见增生,白髓与红髓分界清晰。肺脏组织表面被覆浆膜,未见明显水肿、炎性浸润或纤维结缔组织增生;各级支气管结构完整清晰,支气管上皮细胞形态正常,间质未见明显炎性细胞浸润及纤维组织增生。各组大鼠肾脏组织被膜完整,皮质和髓质分界清晰,肾小管上皮细胞结构正常,未见明显变性坏死。3组大鼠肝脏组织切片均可见局部肝细胞空泡变性,考虑为解剖时动物禁食不够、肝脏内肝糖原含量过高导致,与实验干预无关。 2.8.4 颅骨组织学染色 苏木精-伊红染色:3组颅骨染色切片均可见原缺损部分,空白组缺损位置主要为纤维结缔组织增生占据,可见少量血管生成,未见明显新骨生成;对照组少量新骨生成,实验组缺损区域有明显新骨生成, 两组局部可见不同程度纤维组织增生,纤维细胞呈长梭形,可见新生毛细血管,见图11A。 Masson染色:空白组仅可见结缔组织充填缺损区域,未见明显新骨生成;对照组和实验组缺损区域均可见新骨生成,实验组中心可见新生骨组织较为成熟,且新生骨量较多,见图11B。"
| [1] YAN J, LU X, ZHU X, et al. Effects of miR-26a on Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells by a Mesoporous Silica Nanoparticle - PEI - Peptide System. Int J Nanomedicine. 2020;15:497-511. [2] WANG Z, ZHANG D, HU Z, et al. MicroRNA-26a-modified adipose-derived stem cells incorporated with a porous hydroxyapatite scaffold improve the repair of bone defects. Mol Med Rep. 2015;12:3345-3350. [3] PAQUET J, MOYA A, BENSIDHOUM M, et al. Engineered cell-free scaffold with two-stage delivery of miRNA-26a for bone repair. Ann Transl Med. 2016;4(10):3345-3350. [4] LU J, SONG G, TANG Q, et al. MiR-26a inhibits stem cell-like phenotype and tumor growth of osteosarcoma by targeting Jagged1. Oncogene. 2017;36: 231-241. [5] CAI Y, YU X, HU S, et al. A brief review on the mechanisms of miRNA regulation. Genomics Proteomics Bioinf. 2009;7(4):147-154. [6] WANG T, XU Z. miR-27 promotes osteoblast differentiation by modulating Wnt signaling. Biochem Biophys Res Commun. 2010;402:186-189. [7] SU X, LIAO L, SHUAI Y, et al. MiR-26a functions oppositely in osteogenic differentiation of BMSCs and ADSCs depending on distinct activation and roles of Wnt and BMP signaling pathway. Cell Death Dis. 2015;6(8):e1851. [8] ZHANG R, TANG L, TIAN Y, et al. Cholesterol-modified DP7 enhances the effect of individualized cancer immunotherapy based on neoantigens. Biomaterials. 2020;241:119852. [9] JIN H, PI J, ZHAO Y, et al. EGFR-targeting PLGA-PEG nanoparticles as a curcumin delivery system for breast cancer therapy. Nanoscale. 2017;9: 16365-16374. [10] GARCIA-GARCIA P, REYES R, PEREZ-HERRERO E, et al. Alginate-hydrogel versus alginate-solid system. Efficacy in bone regeneration in osteoporosis. Mater Sci Eng C Mater Biol Appl. 2020;115:111009. [11] YAN H, WANG Z, LI L, et al. DOPA-derived electroactive copolymer and IGF-1 immobilized poly(lactic-co-glycolic acid)/hydroxyapatite biodegradable microspheres for synergistic bone repair. Chem Eng J. 2021;416:1385-8947. [12] LEE JY, KIM SE, YUN YP, et al. Osteogenesis and new bone formation of alendronate-immobilized porous PLGA microspheres in a rat calvarial defect model. J Ind Eng Chem. 2017;52:277-286. [13] YUAN Y, SHI X, GAN Z, et al. Modification of porous PLGA microspheres by poly-l-lysine for use as tissue engineering scaffolds. Colloids Surf B. 2018; 161:162-168. [14] NAM MINH-PHUONG T, NHI THAO-NGOC D, NGHI THI-PHUONG N, et al. Fabrication of injectable bone substitute loading porous simvastatin-loaded poly(lactic-co-glycolic acid) microspheres. Int J Polym Mater Polym Biomater. 2020;69:351-362. [15] SAH H. Protein instability toward organic solvent/water emulsification: implications for protein microencapsulation into microspheres. PDA J Pharm Sci Technol. 1999;53(1):3-10. [16] BATTAFARANO G, ROSSI M, DE MARTINO V, et al. Strategies for Bone Regeneration: From Graft to Tissue Engineering. Int J Mol Sci. 2021;22:1128. [17] ZHANG LY, BI Q, ZHAO C, et al. Recent Advances in Biomaterials for the Treatment of Bone Defects. Organogenesis. 2020;16:113-125. [18] HAMADA T, MATSUBARA H, HIKICHI T, et al. Rat model of an autologous cancellous bone graft. Sci Rep. 2021;11(1):18001. [19] TOURNIER P, GUICHEUX J, PARÉ A, et al. A partially demineralized allogeneic bone graft: in vitro osteogenic potential and preclinical evaluation in two different intramembranous bone healing models. Sci Rep. 2021;11(1):4907. [20] BHARADWAZ A, JAYASURIYA AC. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2020;110:110698. [21] ZIMMERMANN CE, BORNER BI, HASSE A, et al. Donor site morbidity after microvascular fibula transfer. Clin Oral Investig. 2001;5(4):214-219. [22] PEREIRA HF, CENGIZ IF, SILVA FS, et al. Scaffolds and coatings for bone regeneration. J Mater Sci Mater Med. 2020;31:27. [23] SCHMIDT AH. Autologous bone graft: Is it still the gold standard? Injury. 2021; 52 Suppl 2:S18-S22. [24] MANKIN HJ, HORNICEK FJ, RASKIN KA. Infection in massive bone allografts. Clin Orthop Relat Res. 2005;(432):210-216. [25] CHEN CY, CHEN CC, WANG CY, et al. Assessment of the Release of Vascular Endothelial Growth Factor from 3D-Printed Poly-epsilon-Caprolactone/Hydroxyapatite/Calcium Sulfate Scaffold with Enhanced Osteogenic Capacity. Polymers. 2020;12:1455. [26] REN X, WANG Q, LIU C, et al. Osteogenic ability using porous hydroxyapatite scaffold-based delivery of human placenta-derived mesenchymal stem cells. Exp Ther Med. 2021;22:1091. [27] LI C, YANG L, REN X, et al. Grooved hydroxyapatite scaffold modulates mitochondria homeostasis and thus promotes osteogenesis in bone mesenchymal stromal cells. Mol Med Rep. 2020;22:2801-2809. [28] XU T, HE X, CHEN Z, et al. Effect of magnesium particle fraction on osteoinduction of hydroxyapatite sphere-based scaffolds. J Mater Chem B. 2019;7:5648-5660. [29] LI CL, YANG L, REN XH, et al. Groove structure of porous hydroxyapatite scaffolds (HAS) modulates immune environment via regulating macrophages and subsequently enhances osteogenesis. J Biol Inorg Chem. 2019;24:733-745. [30] WEI SA, MA JX, XU L, et al. Biodegradable materials for bone defect repair. Mil Med Res. 2020;7:54. [31] YUAN J, YE Z, ZENG Y, et al. Bifunctional scaffolds for tumor therapy and bone regeneration: Synergistic effect and interplay between therapeutic agents and scaffold materials. Mater Today Bio. 2022;15:100318. [32] WANG B, FENG C, LIU Y, et al. Recent advances in biofunctional guided bone regeneration materials for repairing defective alveolar and maxillofacial bone: A review. Jpn Dent Sci Rev. 2022;58:233-248. [33] WAN Z, ZHANG P, LIU Y, et al. Four-dimensional bioprinting: Current developments and applications in bone tissue engineering. Acta Biomater. 2020;101:26-42. [34] GOMES ME, HOLTORF HL, REIS RL, et al. Influence of the porosity of starch-based fiber mesh scaffolds on the proliferation and osteogenic differentiation of bone marrow stromal cells cultured in a flow perfusion bioreactor. Tissue Eng. 2006;12:801-809. [35] CHENG CH, LAI YH, CHEN YW, et al. Immobilization of bone morphogenetic protein-2 to gelatin/avidin-modified hydroxyapatite composite scaffolds for bone regeneration. J Biomater Appl. 2019;33:1147-1156. [36] CHEN ZY, GAO S, ZHANG YW, et al. Antibacterial biomaterials in bone tissue engineering. J Mater Chem B. 2021;9(11):2594-2612. [37] SU L, FENG Y, WEI K, et al. Carbohydrate-Based Macromolecular Biomaterials. Chem Rev. 2021;121(18):10950-11029. [38] BIONDI M, UNGARO F, QUAGLIA F, et al. Controlled drug delivery in tissue engineering. Adv Drug Deliv Rev. 2008;60:229-242. [39] KANG Y, XU C, MENG LA, et al. Exosome-functionalized magnesium-organic framework-based scaffolds with osteogenic, angiogenic and anti-inflammatory properties for accelerated bone regeneration. Bioact Mater. 2022;18:26-41. [40] JIN S, XIA X, HUANG J, et al. Recent advances in PLGA-based biomaterials for bone tissue regeneration. Acta Biomater. 2021;127: 56-79. [41] BHARADWAZ A, JAYASURIYA AC. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2020;110:110698. [42] Maadani AM, Salahinejad E. Performance comparison of PLA- and PLGA-coated porous bioceramic scaffolds: Mechanical, biodegradability, bioactivity, delivery and biocompatibility assessments. J Control Release. 2022;351:1-7. [43] ZHANG BJ, HAN ZW, DUAN K, et al. Multilayered pore-closed PLGA microsphere delivering OGP and BMP-2 in sequential release patterns for the facilitation of BMSCs osteogenic differentiation. J Biomed Mater Res A. 2018;106:95-105. [44] RAVICHANDRAN R, VENUGOPAL JR, SUNDARRAJAN S, et al. Precipitation of nanohydroxyapatite on PLLA/PBLG/Collagen nanofibrous structures for the differentiation of adipose derived stem cells to osteogenic lineage. Biomaterials. 2012;33:846-855. |
| [1] | Long Guiyue, Li Dongdong, Liao Hongbing. Calcium phosphate cement/poly(lactic-co-glycolic acid) degradation products promote osteoclast differentiation of mouse monocytes [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1193-1198. |
| [2] | Zhang Liang, Han Zekui, Zang Yixin, Han Zhenjia, Wang Xinyu. Design and three-dimensional finite element analysis of 3D printed individualized titanium mesh with a bionic porous spider web-shaped structure [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(30): 4796-4801. |
| [3] | Zhang Xiaoyu, Chen Qi, Yang Xing, Hao Yuefeng. Application of poly(lactic-co-glycolic acid) copolymer microspheres in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(30): 4896-4903. |
| [4] | Zhai Hongjie, Han Guanda, Li Lei, Dong Xiaohui, Jiang Zhiquan, Lou Feiyun. 3D printed polyetheretherketone material for skull defect repair [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 380-384. |
| [5] | Wang Jianghua, Yin Dongfeng, Teng Yong, Wurikaixi·Aiyiti, Wang Xiaofeng, Mareyanmu·Aini, Jiang Houfeng, Patiguli·Aihemaiti, Wang Jing. Optimization of the preparation process of vancomycin-poly(propylene fumarate)/poly(lactic-co-glycolic acid) microspheres by star point design-response surface method [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(16): 2510-2517. |
| [6] | Xia Yu, Sun Jia, Qi Zhengyan, Ma Lin, Ma Wenqian, Niu Jianguo, Wen Yujun. Repair effect of poly(lactic-co-glycolic acid) nanospheres with sustained-release of neurotrophin-3 and ganglioside GD1a on spinal cord injury in rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(16): 2518-2524. |
| [7] | Ji Hangyu, Gu Jun, Xie Linghan, Bao Junping, Peng Xin, Wu Xiaotao. Chitosan/poly(lactic-co-glycolic acid)/polylactic acid scaffold with sustained release of nerve growth factor promotes the differentiation of bone marrow mesenchymal stem cells into neurons [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(25): 3974-3979. |
| [8] | Huang Jie, Ren Jing, Peng Pairan, Mu Yandong. Treatment of periodontitis in rats with a novel temperature-sensitive gel with immunomodulatory peptide [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(22): 3514-3520. |
| [9] | Hu Junxian, Zhao Deying, Wang Lei, Huang Minghuo, Wu Yalan, Chen Jincao, Liu Zheng. Complication comparison and application improvement of 3D-printed plastic polyetherketone and titanium mesh cranioplasty [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(21): 3327-3331. |
| [10] | Fan Haixia, Tan Qingkun, Wang Hong, Cheng Huanzhi, Liu Xue, Ching-chang Ko, Geng Haixia. Rabbit skull defects repaired by the hydroxyapatite/geltin scaffold combined with bone marrow mesenchymal stem cells and umbilical vein endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(10): 1495-1499. |
| [11] |
Wang Dexin, Xu Zhanwu, Pei Guoxian.
Osteogenesis of bone marrow mesenchymal stem cells on hydroxyapatite/icariin/poly(lactic-co-glycolic acid) scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(25): 3974-3980. |
| [12] | Zong Qiang, Xu Yanan, Qu Tianyi, Li Lijun, Hai Miti·Abuduaini, Ni Dongkui. Magnetic verapamil nanoparticles promote peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(34): 5425-5429. |
| [13] | Zhu Xianhua, Chen Feng, Liu Chongdong, Zhou Wei, Tang Xinhua . Dextran/poly(lactic-co-glycolic acid) microspheres combined with three growth factors promote neovascularization in ischemic lower limbs of rats [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(26): 4142-4147. |
| [14] | Chen Jiao, Shu Liping, Li Xuanze, Liu Qin, Wu Ying, Liu Yin, Wang Weiyu, Liu Jun, Ye Chuan, Ma Minxian. Construction of tissue engineered bone by poly(lactic-co-glycolic acid) scaffold carrying human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(26): 4109-4114. |
| [15] | Zhang Wen-yuan, Yang Ya-dong, Li Ying, Zhang Ke-ji, Fang Guo-jian, Tang Liang, Li Yue-zhong, Wang Han, Lu Ming-yang. Silk/poly(lactic-co-glycolic acid) scaffold degradation fluid and proliferation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(25): 4676-4683. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||