Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (6): 977-984.doi: 10.12307/2023.269
Mesenchymal stem cells for allergic rhinitis: a meta-analysis based on animal experiments
Yan Le1, Zhang Huiping1, Dai Lintong2
- 1School of Medicine and Life Sciences/Affiliated Reproductive and Maternity Hospital, Chengdu University of Traditional Chinese Medicine, Chengdu 610000, Sichuan Province, China; 2Department of Otolaryngology, Head and Neck Surgery, Affiliated Hospital of Panzhihua University, Panzhihua 617000, Sichuan Province, China
-
Received:2022-03-24Accepted:2022-05-17Online:2023-02-28Published:2022-08-12 -
Contact:Dai Lintong, Master, Associate chief physician, Associate professor, Master’s supervisor, Department of Otolaryngology, Head and Neck Surgery, Affiliated Hospital of Panzhihua University, Panzhihua 617000, Sichuan Province, China -
About author:Yan Le, Master candidate, School of Medicine and Life Sciences/Affiliated Reproductive and Maternity Hospital, Chengdu University of Traditional Chinese Medicine, Chengdu 610000, Sichuan Province, China -
Supported by:Special Project of Traditional Chinese Medicine Scientific Research of Sichuan Provincial Administration of Traditional Chinese Medicine, No. 458 (to DLT)
CLC Number:
Cite this article
Yan Le, Zhang Huiping, Dai Lintong. Mesenchymal stem cells for allergic rhinitis: a meta-analysis based on animal experiments[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 977-984.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

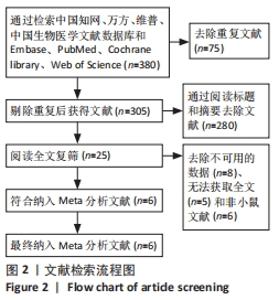
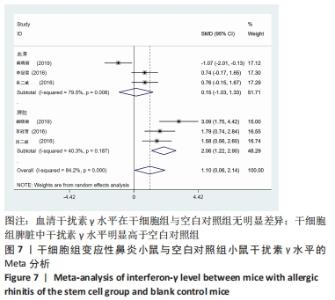
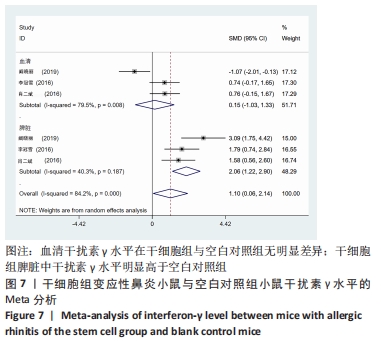
2.4 Meta分析结果 2.4.1 变应性鼻炎症状 总共有5篇文献提到了过敏性鼻炎的症状[15-16,18-20],其中3篇分别提到了喷嚏次数和揉鼻次数[15,18,20],其中干细胞组小鼠21只,动物模型对照组小鼠20只,对各研究进行异质性检验,I2=93.7%,故采用随机效应模型进行Meta分析,喷嚏次数亚组结果提示:干细胞组的喷嚏次数明显少于动物模型对照组,且差异有显著性意义[SMD=-9.83,95%CI(-17.45,-2.22),P=0.011],揉鼻次数亚组结果显示:干细胞组的揉鼻次数明显少于动物模型对照组,差异有显著性意义[SMD=-13.27,95%CI(-25.04,-1.49),P=0.027],见图3。"

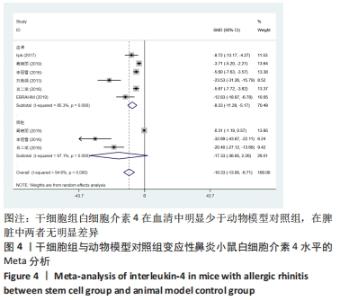
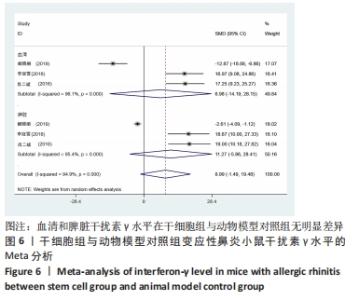
2.4.2 白细胞介素4水平 干细胞组vs.动物模型对照组:6篇文献提到了血清白细胞介素4水平[15-20],其中有3篇提到了脾脏白细胞介素4水平[16- 17,19],干细胞组小鼠51只,动物模型对照组小鼠50只,对各研究进行异质性检验,I2=94.6%,故采用随机效应模型进行Meta分析,在血清白细胞介素4水平亚组中分析结果显示:干细胞组的白细胞介素4水平明显低于动物模型对照组,差异有显著性意义[SMD=-8.23,95%CI(-11.28,-5.17),P=0],脾脏白细胞介素4水平分析结果显示:白细胞介素4水平在干细胞组与动物模型对照组之间并没有明显差异[SMD=-17.33,95%CI(-36.85,2.20),P=0.082],见图4。 "

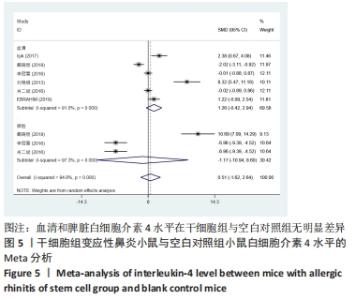
干细胞组vs.空白对照组:6篇文献提到了血清白细胞介素4水平[15-20],其中有3篇提到了脾脏白细胞介素4水平[16-17,19],干细胞组小鼠51只,动物模型对照组小鼠50只,对各研究进行异质性检验,I2=94.8%,故采用随机效应模型进行Meta分析,血清白细胞介素4水平亚组分析结果显示:白细胞介素4水平在干细胞组与空白对照组之间并没有明显差异[SMD=1.26,95%CI(-0.42,2.94),P=0.142],脾脏白细胞介素4水平亚组分析结果显示:白细胞介素4水平在干细胞组与空白对照组之间并没有明显差异[SMD=-1.17,95%CI(-10.94,8.60),P=0.815],见图5。 "

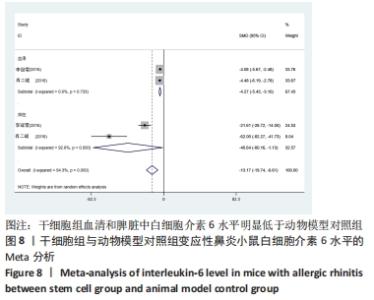
2.4.4 白细胞介素6水平 干细胞组vs.动物模型对照组:共有2篇文献提到了白细胞介素6水平[16-17],这2篇中均提到了血清中与脾脏中的白细胞介素6水平,其中干细胞组小鼠20只,动物模型对照组小鼠20只。对各研究进行异质性检验,I2=94.3%,故采用随机效应模型进行Meta分析。血清白细胞介素6亚组分析结果显示:干细胞组白细胞介素6水平明显低于动物模型对照组,且差异有显著性意义[SMD=-4.27,95%CI (-5.43,-3.10),P=0.035],脾脏白细胞介素6亚组分析结果显示:干细胞组白细胞介素6水平低于动物模型对照组[SMD= -40.64,95%CI(-80.16,-1.13),P=0],见图8。 "

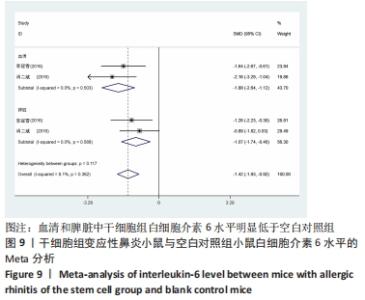
干细胞组vs.空白对照组:共有2篇文献提到了白细胞介素6水平[16-17],这2篇中均提到了血清中与脾脏中的白细胞介素6水平,其中干细胞组小鼠20只,空白对照组小鼠20只。对各研究进行异质性检验,I2=6.1%,故采用固定效应模型进行Meta分析。血清白细胞介素6亚组分析结果显示:干细胞组白细胞介素6水平明显低于空白对照组,且差异有显著性意义[SMD=-1.88,95%CI(-2.64,-1.12),P=0],脾脏白细胞介素6亚组分析结果显示:干细胞组白细胞介素6水平明显低于空白对照组,且差异有显著性意义[SMD=-1.07,95%CI(-1.74,-0.40),P=0.002],见图9。 "


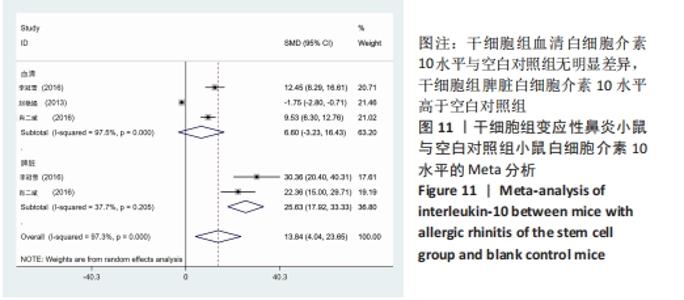
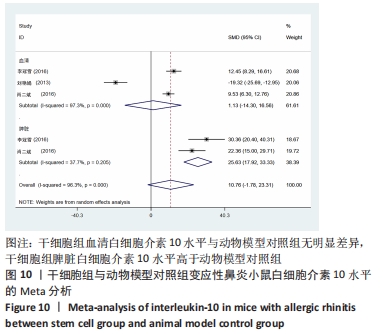
2.4.5 白细胞介素10水平 干细胞组vs.动物模型对照组:共有3篇文献提到了血清白细胞介素10水平[15-17],其中2篇提到了脾脏白细胞介素10水平[16-17],干细胞组小鼠30只,动物模型对照组小鼠30只,对各研究进行异质性检验,I2=96.3%,故采用随机效应模型进行Meta分析,血清白细胞介素10水平亚组分析结果显示:干细胞组与动物模型对照组之间无明显差异[SMD=1.13,95%CI(-14.30,16.56),P=0.886],脾脏白细胞介素10水平亚组分析结果显示:干细胞组脾脏白细胞介素10水平明显高于动物模型对照组[SMD=25.63,95%CI(17.92,33.33),P=0],见图10。 "
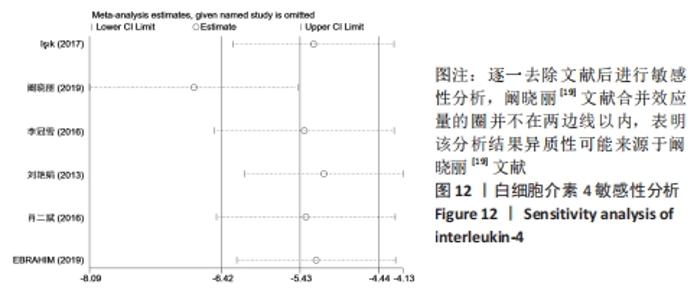
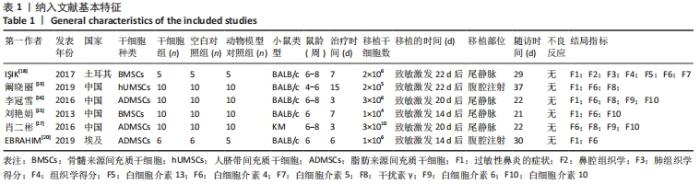
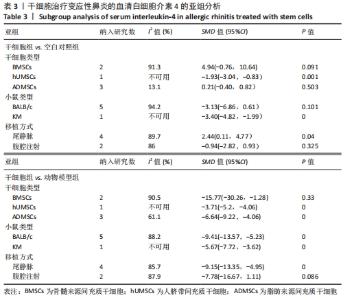
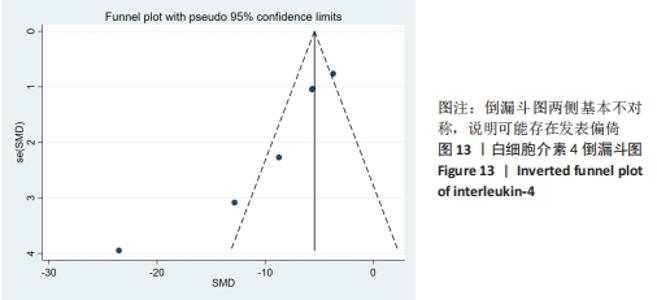
小鼠类型:干细胞组BALB/c类型小鼠血清白细胞介素4水平与空白对照组未见明显的差异[SMD=-3.13,95%CI(-6.86,0.61),P=0.101];干细胞组KM类型小鼠血清白细胞介素4水平较空白对照组明显降低[SMD=-3.40,95%CI(-4.82,-1.99),P=0],干细胞与动物模型组比较见表3。 注射方式:经尾静脉注射干细胞组白细胞介素4水平明显高于空白对照组[SMD=2.44,95%CI(0.11,4.77),P=0.040];经腹腔注射干细胞组白细胞介素4水平与空白对照组无明显差异[SMD=-0.94,95%CI(-2.82,0.93),P=0.325],干细胞与动物模型组比较见表3。 2.5 敏感性分析 采用stata15.0软件对白细胞介素4逐一去除文献进行敏感性分析,当去除阚晓丽[19]文献时发现合并效应量的圈并不在两边线内,表明血清白细胞介素4的分析结果异质性可能来源于阚晓丽[19]的文献,见图12。 "

| [1] GREINER AN, HELLINGS PW, ROTIROTI G, et al. Allergic rhinitis. Lancet. 2011; 378 (9809): 2112-2122. [2] BROŻEK JL, BOUSQUET J, AGACHE I, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017;140(4):950-958. [3] SCHMITT J, STADLER E, KÜSTER D, et al. Medical care and treatment of allergic rhinitis: a population-based cohort study based on routine healthcare utilization data. Allergy. 2016;71(6):850-858. [4] BOUSQUET J, SCHÜNEMANN HJ, TOGIAS A, et al. Next-generation Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines for allergic rhinitis based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and real-world evidence. J Allergy Clin Immunol. 2020;145(1):70-80. [5] KAWAUCHI H, YANAI K, WANG DY, et al. Antihistamines for Allergic Rhinitis Treatment from the Viewpoint of Nonsedative Properties. Int J Mol Sci. 2019; 20(1):213. [6] DIERICK BJH, VAN DER MOLEN T, FLOKSTRA-DE BLOK BMJ, et al. Burden and socioeconomics of asthma, allergic rhinitis, atopic dermatitis and food allergy. Expert Rev Pharmacoecon Outcomes Res. 2020;20(5):437-453. [7] SCHULER IV CF, MONTEJO JM. Allergic Rhinitis in Children and Adolescents. Pediatr Clin North Am. 2019;66(5):981-993. [8] ZHANG Y, ZHANG L. Increasing Prevalence of Allergic Rhinitis in China. Allergy Asthma Immunol Res. 2019;11(2):156-169. [9] OKUBO K, KURONO Y, ICHIMURA K, et al. Japanese guidelines for allergic rhinitis 2017. Allergol Int. 2017;66(2):205-219. [10] ANKRUM JA, ONG JF, KARP JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252-260. [11] DING DC, CHANG YH, SHYU WC, et al. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015;24(3):339-347. [12] SAMSONRAJ RM, RAGHUNATH M, NURCOMBE V, et al. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl Med. 2017;6(12):2173-2185. [13] FU X, LIU G, HALIM A, et al. Mesenchymal Stem Cell Migration and Tissue Repair. Cells. 2019;8(8):784. [14] HOOIJMANS CR, ROVERS MM, DE VRIES RB, et al. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014; 14:43. [15] 刘艳娟. BMSCs的免疫负调节性对变应性鼻炎小鼠炎症反应的影响[D].沈阳:中国医科大学,2013. [16] 李冠雪,刘艳慧,申聪香,等.脂肪来源间充质干细胞调控变应性鼻炎小鼠T细胞免疫状态的研究[J].中华耳鼻咽喉头颈外科杂志,2016,51(1):50-56. [17] 肖二彬,赵宝建,张驰.脂肪间充质干细胞可调节变应性鼻炎T细胞的免疫状态[J].中国组织工程研究,2016,20(10): 1373-1381. [18] IŞIK S, KARAMAN M, ADAN A, et al. Intraperitoneal mesenchymal stem cell administration ameliorates allergic rhinitis in the murine model. Eur Arch Otorhinolaryngol. 2017;274(1):197-207. [19] 阚晓丽.人脐带间充质干细胞治疗小鼠变应性鼻炎的疗效与机制研究[D].昆明:昆明医科大学,2019. [20] EBRAHIM N, MANDOUR YMH, FARID AS, et al. Adipose Tissue-Derived Mesenchymal Stem Cell Modulates the Immune Response of Allergic Rhinitis in a Rat Model. Int J Mol Sci. 2019;20(4):873. [21] GALLI SJ, TSAI M, PILIPONSKY AM. The development of allergic inflammation. Nature. 2008;454(7203):445-454. [22] 戴玉洋,赵秀丽.间充质干细胞治疗变应性鼻炎的研究进展[J].中国临床药理学杂志,2019,35(5):482-485. [23] LIU H, LI R, LIU T, et al. Immunomodulatory Effects of Mesenchymal Stem Cells and Mesenchymal Stem Cell-Derived Extracellular Vesicles in Rheumatoid Arthritis. Front Immunol. 2020;11:1912. [24] CHEN Y, YU Q, HU Y, et al. Current Research and Use of Mesenchymal Stem Cells in the Therapy of Autoimmune Diseases. Curr Stem Cell Res Ther. 2019; 14(7):579-582. [25] ZHAO L, CHEN S, YANG P, et al. The role of mesenchymal stem cells in hematopoietic stem cell transplantation: prevention and treatment of graft-versus-host disease. Stem Cell Res Ther. 2019;10(1):182. [26] TROMBITAȘ VE, NAGY AA, BERCE C, et al. The Role of Mesenchymal Stem Cells in the Treatment of a Chronic Rhinosinusitis-An In Vivo Mouse Model. Microorganisms. 2021;9(6):1182. [27] DAI YY, NI SY, MA K, et al. Stem cells from human exfoliated deciduous teeth correct the immune imbalance of allergic rhinitis via Treg cells in vivo and in vitro. Stem Cell Res Ther. 2019;10(1):39. [28] CHO KS, PARK HK, PARK HY, et al. IFATS collection: Immunomodulatory effects of adipose tissue-derived stem cells in an allergic rhinitis mouse model. Stem Cells. 2009;27(1):259-265. [29] PARK IS, KIM JH, BAE JS, et al. The Supernatant of Tonsil-Derived Mesenchymal Stem Cell Has Antiallergic Effects in Allergic Rhinitis Mouse Model. Mediators Inflamm. 2020;2020:6982438. [30] FU QL, CHOW YY, SUN SJ, et al. Mesenchymal stem cells derived from human induced pluripotent stem cells modulate T-cell phenotypes in allergic rhinitis. Allergy. 2012;67(10):1215-1222. [31] SUN YQ, DENG MX, HE J, et al. Human pluripotent stem cell-derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem Cells. 2012;30(12):2692-2699. |
| [1] | Dang Yi, Du Chengyan, Yao Honglin, Yuan Nenghua, Cao Jin, Xiong Shan, Zhang Dingmei, Wang Xin. Hormonal osteonecrosis and oxidative stress [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1469-1476. |
| [2] | Wang Yanjin, Zhou Yingjie, Chai Xubin, Zhuo Hanjie. Meta-analysis of the efficacy and safety of 3D printed porous titanium alloy fusion cage in anterior cervical discectomy and fusion [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1434-1440. |
| [3] | Jiang Xiaocheng, Shi Lu, Wang Yinbin, Li Qiujiang, Xi Chuangzhen, Ma Zefeng, Cai Lijun. Systematical evaluation of bone fusion rate after interbody fusion in patients with osteoporosis and lumbar degenerative disease treated with teriparatide [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1427-1433. |
| [4] | Xue Ting, Zhang Xinri, Kong Xiaomei. Mesenchymal stem cell therapy for pneumoconiosis using nanomaterials combined with multi-modal molecular imaging [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1133-1140. |
| [5] | Li Wenjie, You Aijia, Zhou Junli, Fang Sujuan, Li Chun. Effects of different dressings in the treatment of burn wounds: a network meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1141-1148. |
| [6] | Liu Wentao, Feng Xingchao, Yang Yi, Bai Shengbin. Effect of M2 macrophage-derived exosomes on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 840-845. |
| [7] | Long Yanming, Xie Mengsheng, Huang Jiajie, Xue Wenli, Rong Hui, Li Xiaojie. Casein kinase 2-interaction protein-1 regulates the osteogenic ability of bone marrow mesenchymal stem cells in osteoporosis rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 878-882. |
| [8] | Cui Lianxu, Jiang Wenkang, Lu Dahong, Xu Junrong, Liu Xiaocui, Wang Bingyun. Clinical-grade human umbilical cord mesenchymal stem cells affect the improvement of neurological function in rats with traumatic brain injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 835-839. |
| [9] | Li Qicheng, Deng Jin, Fu Xiaoyang, Han Na. Effects of bone marrow mesenchymal stem cells-derived exosomes on hypoxia-treated myoblasts [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 853-859. |
| [10] | Wang Min, Yin Xiushan, Wang Yingxi, Zhang Yan, Zhao Long, Xia Shuyue. Inhalation of bone marrow mesenchymal stem cells-derived exosomes alleviates inflammatory injury in chronic obstructive pulmonary disease [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 827-834. |
| [11] | Huang Guijiang, Ji Yuwei, Zhao Xin, Yang Yi, Zhao Yulan, Wang Peijin, Tang Wei, Jiao Jianlin. Effect and mechanism of different administration routes of placenta-derived mesenchymal stem cells in the treatment of tree shrews with osteoporotic fracture [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 909-914. |
| [12] | Zhang Qijian, Xu Ximing. Acquisition and application of ectodermal mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 928-934. |
| [13] | Xiong Juan, Guan Yalin, Yang Yutong, Wang Fan, Liu Zhongshan. Application of stem cells to skin anti-aging [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 948-954. |
| [14] | Xu Qijing, Yang Yichun, Lei Wei, Yang Ying, Yu Jiang, Xia Tingting, Zhang Meng, Zhang Tao, Zhang Qian. Advances and problems in cell-free treatment of diabetic skin chronic wounds [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 962-969. |
| [15] | Chen Guanting, Zhang Linqi, Li Qingru. Meta-analysis of the value of exosomal miRNA for the diagnosis of chronic kidney disease [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 970-976. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||