Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (6): 962-969.doi: 10.12307/2023.241
Previous Articles Next Articles
Advances and problems in cell-free treatment of diabetic skin chronic wounds
Xu Qijing1, Yang Yichun2, Lei Wei2, Yang Ying2, Yu Jiang3, Xia Tingting2, Zhang Meng3, Zhang Tao2, 3, Zhang Qian1
- 1Department of Human Anatomy, School of Basic Medicine, Zunyi Medical University, Zunyi 563000, Guizhou Province, China; 2Department of Dermatology, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China; 3Key Laboratory of Cell Engineering of Guizhou Province, Affiliated Hospital of Zunyi Medical University, Zunyi 563003, Guizhou Province, China
-
Received:2022-02-07Accepted:2022-04-18Online:2023-02-28Published:2022-08-12 -
Contact:Zhang Qian, Professor, Master’s supervisor, Department of Human Anatomy, School of Basic Medicine, Zunyi Medical University, Zunyi 563000, Guizhou Province, China Zhang Tao, Professor, Master’s supervisor, Department of Dermatology, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China; Key Laboratory of Cell Engineering of Guizhou Province, Affiliated Hospital of Zunyi Medical University, Zunyi 563003, Guizhou Province, China -
About author:Xu Qijing, Master candidate, Department of Human Anatomy, School of Basic Medicine, Zunyi Medical University, Zunyi 563000, Guizhou Province, China -
Supported by:the Science and Technology Program of Zunyi City, No. (2019)90 (to ZT)
CLC Number:
Cite this article
Xu Qijing, Yang Yichun, Lei Wei, Yang Ying, Yu Jiang, Xia Tingting, Zhang Meng, Zhang Tao, Zhang Qian. Advances and problems in cell-free treatment of diabetic skin chronic wounds[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 962-969.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
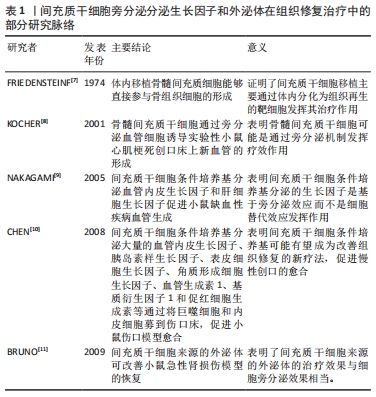
2.1 糖尿病足溃疡慢性创面的病理生理学 伤口愈合过程可以定义为复杂的级联反应,依赖于多种组织修复机制,以最佳方式恢复组织完整性和功能,包括凝血、炎症、基质合成、血管生成和组织重塑。然而,在非愈合性慢性创面中,如糖尿病足溃疡,上述高度协调的过程被持续的炎症状态和因活性氧产生过多而导致创面长期微循环障碍,降低创面愈合质量。糖尿病足溃疡是糖尿病慢性严重的并发症之一,常因长期的血糖控制不佳、局部创伤与感染等多重因素诱发,造成创面炎症期延迟、血管生成受损等,使得慢性创面迁延不愈。高血糖是糖尿病最主要的特征之一,持续的高血糖使得内皮代谢和功能发生异常,导致一系列微观和宏观循环功能障碍,最终影响血管生成过程[13]。由于长期暴露于高糖的环境下,内皮细胞失去完整性,变得容易脱落和凋亡[14],从而导致糖尿病足溃疡创面愈合进程发生异常甚至停滞。持续的高血糖还会导致多种晚期糖基化终产物,进而诱导M1巨噬细胞的增多,释放多种促炎性因子,破坏皮肤创面愈合过程[15]。不仅如此,慢性炎症也是破坏糖尿病足溃疡愈合过程的最常见原因之一。葡萄糖和游离脂肪酸浓度在各种组织中持续升高可诱发炎症反应过程。创面慢性炎症的标志是免疫细胞(如巨噬细胞、淋巴细胞和浆细胞)浸润到创面部位,并释放导致创面愈合不佳的促炎性分子[16]。另外,巨噬细胞从促炎的M1表型极化为抗炎的M2表型有利于抑制创面的炎症反应,为创面愈合产生积极作用。然而,研究表明糖尿病足溃疡中M1巨噬细胞向M2巨噬细胞表型极化存在困难,表现为M1巨噬细胞在创面的持续性浸润,分泌大量的促炎因子,进一步加剧了创面的炎症反应[17]。糖尿病足溃疡还伴随着生长因子的分泌和活性降低,内皮祖细胞的募集减少,成纤维细胞和角质形成细胞的增殖和迁移受损,以及胶原基质的形成受损[18]。 在正常的急性伤口愈合中,损伤部位会向角质形成细胞、成纤维细胞、内皮细胞、巨噬细胞和血小板等释放多种细胞因子和趋化因子,介导与组织修复相关细胞迁移到受损区域参与损伤修复的过程[19]。令人遗憾的是,在糖尿病足溃疡创面中,由于局部长期的高血糖、缺氧和慢性炎症使得角质形成细胞和成纤维细胞等增殖、迁移和分化过程严重受损,细胞因子和生长因子之间表达不平衡,机体的自我修复能力明显下降,造成慢性伤口难以愈合[20]。 2.2 生长因子与糖尿病足溃疡创面愈合 生长因子是一类可溶性的信号蛋白,它是参与细胞生长、增殖、迁移和分化的天然多肽[21],影响多种细胞类型的增殖和迁移、血管的生成以及成纤维细胞和炎症细胞的趋化,从而参与伤口愈合过程的不同阶段,促进细胞外基质产生和重塑。生长因子与其受体的特异性结合激活了调节亚细胞生理学和细胞功能各个方面的细胞内信号转导途径,并表现出不同的细胞反应[22]。在启动和维持伤口愈合的不同阶段中,生长因子起着关键作用,生长因子受体的下调和生长因子的快速降解,都会导致创面愈合延迟[23]。在动物性糖尿病创面模型实验中,发现对创面愈合至关重要的生长因子(包括血管内皮生长因子、转化生长因子β等)的减少[24]。有学者归纳了几种对创面愈合有积极作用的生长因子,如血管内皮生长因子、血源性血小板生长因子(blood derived platelet growth factor,PDGF)、转化生长因子β以及成纤维细胞生长因子等,这些生长因子可诱导角质形成细胞和成纤维细胞的增殖,促使肉芽组织中毛细血管的形成,并调节细胞外基质沉积和损伤区域的组织结构重建[25]。由于生长因子在创面愈合中的作用及其在慢性创面中的不足,因此,对外源性的生长因子或干细胞分泌的生长因子在创面局部施用是促进创面愈合的一种有希望的策略,见表2。 "
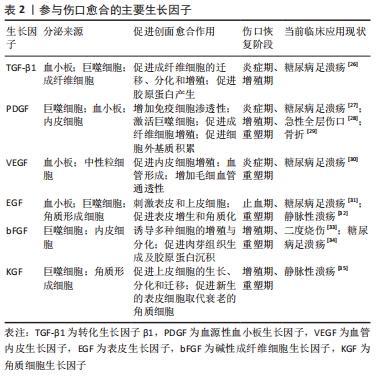
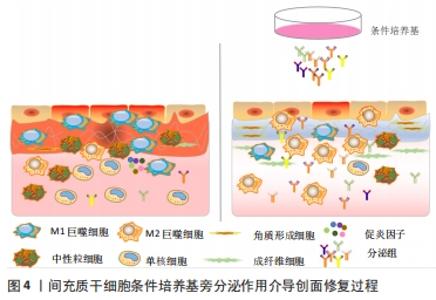
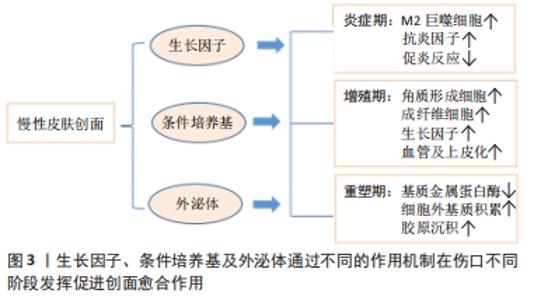
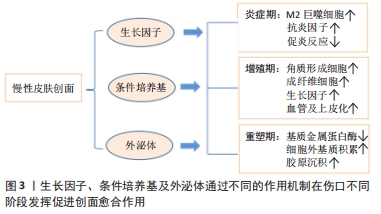
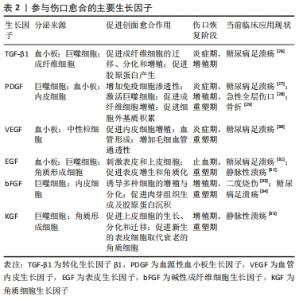
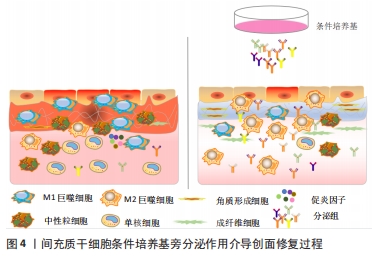
PDGF是创面愈合中被广泛研究的生长因子之一,在伤口愈合的各个阶段都可刺激成纤维细胞、中性粒细胞、单核细胞向损伤部位的迁移[25]。重组人PDGF-BB(Becaplermin)是目前被美国食品及药品监督管理局(Food and Drug Administration,FDA)批准的唯一用于治疗慢性创面的生长因子,并且已成功用于糖尿病足溃疡治疗当中[27]。将PDGF联合含有抗生素的特定生物支架进行干预,同样也有效促进了糖尿病大鼠感染的创面恢复[36]。血管内皮生长因子是另一种在创面愈合过程中起重要作用的生长因子,它能够激活内皮细胞和诱导新生血管的形成,并且是生长因子家族中促进内皮细胞增殖最强的生长因子,也是决定创面血管新生的关键因子[37]。血管内皮生长因子通过与内皮细胞上的酪氨酸激酶受体VEGFR-2结合发挥血管生成作用[38]。它主要涉及蛋白酶对现有的血管细胞外基质的降解,并导致毛细血管内皮细胞的迁移和增殖。KULWAS等[39]研究表明,2型糖尿病合并足溃疡的患者与无足溃疡的糖尿病患者相比,血清中的VEGFR-2和VEGF-A水平均降低,并导致了血管形成受阻,成为伤口愈合延迟的原因。然而,GANGADARAM等[40]研究表明骨髓间充质干细胞来源的外泌体可通过激活内皮细胞的VEGFR-2,介导VEGF-A的合成增加,促进了小鼠后肢糖尿病足溃疡处的内皮细胞的增殖、迁移和创面新生血管生成,从而改善了缺血后肢的血液灌注与创面修复。 此外,为解决外源性生长因子因半衰期短及稳定性低等影响治疗效果的问题,将生长因子与生物支架结合作为药物系统载体输送来提高治疗效果逐渐被应用于组织缺损修复当中。最近一项研究表明,将载有血管内皮生长因子和基质细胞衍生因子1α共修饰的胶原支架植入糖尿病大鼠皮肤创伤模型后,观察到两种重组蛋白从胶原支架上持续释放,不仅在促进血管生成方面显示出协同效应,同时在短期内减少了炎症,进而促进细胞增殖、血管再生、再上皮化和细胞外基质积累[41]。WHITE等[42]通过利用VEGF-A、PDGF-BB和肝素结合表皮生长因子联合治疗糖尿病小鼠模型,显示“三联疗法”的联合作用比单独使用生长因子效果更佳,表现为M1巨噬细胞和效应T细胞数量明显减少,并且在局部损伤部位停留的时间明显长于其他生长因子。碱性成纤维细胞生长因子被认为是一种有效的有丝分裂原,能够促进成纤维细胞增殖和新生血管形成,也是加速伤口愈合的关键因子。在一项临床研究中,MATSUMOTO等[34]采用碱性成纤维细胞生长因子浸渍明胶片对急性或慢性溃疡患者进行外敷治疗,发现8例患者中有6例不同类型慢性溃疡患者的皮肤创面可实现完全上皮化,并证实了碱性成纤维细胞生长因子具有促进成纤维细胞增殖、胶原成熟和诱导新生血管生成的作用。研究表明,在人脐带间充质干细胞条件培养基(hUMSC-CM)里检测到与伤口愈合相关旁分泌生长因子碱性成纤维细胞生长因子及血管内皮生长因子的存在,并显著促进大鼠模型创面的再上皮化和胶原蛋白形成[43]。脂肪来源的干细胞条件培养基(ADSC-CM)通过旁分泌转化生长因子β,体外诱导成纤维细胞向肌成纤维的转化[44]。并且,XIN等[45]体外研究发现,人包皮来源真皮干/祖细胞条件培养基(hFDSPC-CM)通过体外转化生长因子β/Smad信号通路促进成纤维细胞的胶原蛋白生成。 2.3 条件培养基与糖尿病足溃疡创面愈合 近些年的研究表明,间充质干细胞的治疗作用可能是通过旁分泌方式分泌可溶性因子介导的,通过招募和改善靶组织中的天然细胞活性来强化组织修复和再生的内源性机制[5-6]。条件培养基是指含有多种细胞分泌体和游离细胞因子的细胞上清液,其发挥治疗作用类似细胞治疗中的旁分泌效应。目前间充质干细胞条件培养基在伤口愈合方面的潜力已逐步得到研究证实,因此可能作为基于干细胞治疗的更好替代解决方案之一[46]。间充质干细胞条件培养基中干细胞分泌的旁分泌因子(称为分泌组)中含有多种细胞因子、趋化因子和生长因子,如血管内皮生长因子、表皮生长因子、角质细胞生长因子、基质细胞衍生因子1等[47],对组织再生修复有多重积极影响。研究表明,间充质干细胞条件培养基通过刺激与创面愈合相关的细胞和细胞因子的迁移和增殖,在创面内诱导新生血管形成、加速上皮化和抑制炎症,继而促进皮肤创面愈合[48],见图4。 "
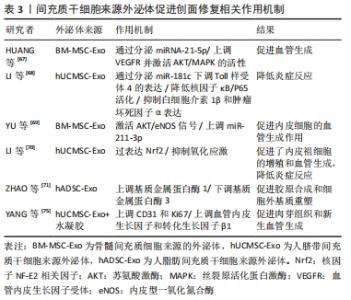
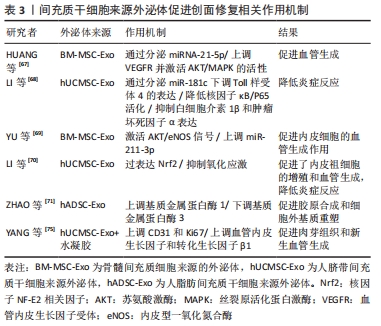
慢性创面愈合依赖良的好肉芽组织形成和再上皮化,其中角质形成细胞及成纤维细胞的增殖、迁移和再上皮化是创面愈合的基础。先前研究表明,在糖尿病病理条件下,角质形成细胞和成纤维细胞的生物学功能明显受损,其特征是细胞过早衰老、形态异常,增殖和迁移减少,以及分泌生物活性因子和产生细胞外基质的能力降低[49-50]。然而,有研究者发现,人骨髓间充质干细胞条件培养基可使得表皮生长因子和碱性成纤维细胞生长因子上调,从而改善糖尿病微环境中成纤维细胞的细胞行为[51]。新近的一项研究也表明,脂肪干细胞条件培养基可以通过促进转化生长因子β1、血管内皮生长因子的分泌以及成纤维细胞的增殖、迁移和分化来加速糖尿病大鼠的伤口愈合[52]。JOSEPH等[53]将犬、山羊和豚鼠来源的骨髓间充质干细胞条件培养基分别治疗了豚鼠皮肤创面模型后发现,3种不同来源的骨髓间充质干细胞条件培养基均可促进创面血管形成和胶原蛋白的沉积,促进了创面的愈合,三者的治疗效果相当,同时也初步证明了骨髓间充质干细胞条件培养基可以用于同种异体或者异种应用。 条件培养基的获取方式和条件也对创面愈合的效果有着不同的影响。有研究者在3种不同的培养条件下,即常氧、标准和缺氧条件下培养羊膜间充质干细胞获得的条件培养基联合水凝胶局部应用于糖尿病小鼠创面模型治疗后,发现缺氧条件下的羊膜间充质干细胞条件培养基水凝胶更能促进血管生成、加速上皮化和抑制炎症,从而提高糖尿病小鼠的创面闭合率[54]。在导致糖尿病足溃疡的众多因素中,内皮细胞功能障碍导致的血管生成受损至关重要[55]。然而通过局部注射脐带间充质干细胞条件培养基可以促进糖尿病小鼠创面模型的修复,是由于脐带间充质干细胞条件培养基分泌的前列腺素E2通过重塑巨噬细胞表型改善血管内皮细胞功能障碍,以及分泌白细胞介素10和血管内皮生长因子改善了血管内皮细胞的局部微环境,并促进糖尿病小鼠模型创面伤口愈合[56]。内皮祖细胞作为参与血管生成的关键细胞,在血管生成和伤口愈合中起着重要作用,内皮祖细胞向伤口部位的募集取决于基质细胞衍生因子1α的上调[57]。 GALLAGHER等[58]报道糖尿病小鼠创面内基质细胞衍生因子1α表达降低,导致内皮祖细胞向伤口归巢减少,创面血管生成减少。在1型糖尿病大鼠MRSA感染伤口模型中,应用骨髓间充质干细胞条件培养基干预后,伤口处的基质细胞衍生因子1α表达增多,并且中性粒细胞和巨噬细胞数量下降、成纤维细胞计数增加且血管生成增多,炎症得到控制,大鼠创面模型从而得到显著改善[59]。也有研究采用骨髓间充质干细胞条件培养基治疗兔耳增生性瘢痕,发现其可以调控创伤愈合的增殖和重塑阶段,通过上调基质金属蛋白酶1表达而下调基质金属蛋白酶13表达,转化生长因子β1刺激和活化成纤维细胞,调节细胞增殖和组织重塑[60]。 2.4 外泌体与糖尿病足溃疡创面愈合 外泌体(exosomes)是由活细胞分泌的一种纳米级的细胞外囊泡,大小于介于30-200 nm之间。几乎所有的细胞都能够产生外泌体,且为脂质双层膜性结构,富含多种生物活性分子,包括蛋白质、DNA、mRNA、微小RNA (microRNAs,miRNAs)、脂类等[61]。经释放后的外泌体,可以显现出具有多样的活性物质,如重塑细胞外基质及向其他细胞传递信号和分子,成为细胞间传递信号的天然载体,通过与靶细胞相互作用激活各种信号通路发挥生物学效应[62]。随着对外泌体研究的不断深入,发现其在调节炎症反应、细胞免疫、促进血管再生、控制细胞的增殖和凋亡、延缓神经退行性变等生物学过程中均发挥着重要的作用[63]。伤口愈合是一系列复杂的分子和细胞事件过程,如细胞增殖和迁移、血管生成、细胞外基质沉积与组织重塑[64],从外泌体中提取的不同类型的活性分子可参与上述过程[65]。此外,具有脂质双分子层的外泌体还可以避免被蛋白水解,从而确保了外泌体中的活性成分对靶细胞(例如内皮细胞、角质形成细胞和成纤维细胞)发挥作用[66]。外泌体特别是作为干细胞旁分泌的亚细胞成分,在组织修复和再生方面有着潜在的治疗应用优势。 外泌体中的miRNA在血管生成中的作用越来越受到关注。HUANG等[67]研究表明骨髓间充质干细胞来源的外泌体通过分泌miRNA-21-5p上调血管内皮生长因子受体以及丝氨酸/苏氨酸激酶(AKT)和丝裂原活化蛋白激酶(MAPK)的活性,进而促进糖尿病大鼠溃疡模型创面的血管生成和组织损伤与修复。此外,LI等[68]将人脐带间充质干细胞来源外泌体通过尾静脉注入严重烧伤引起的过度炎症反应大鼠模型,人脐带间充质干细胞来源外泌体通过分泌miR-181c降低了大鼠Toll样受体4(TLR4)的表达,同时下调NF-κB/P65,从而抑制白细胞介素1β和肿瘤坏死因子等炎症因子的释放。在一项临床实验中,YU等[69]通过阿托伐他汀(ATV)预处理后的骨髓间充质干细胞分泌的外泌体显示能够激活AKT/eNOS信号传导机制,该机制通过上调miR-211-3p进一步启动内皮细胞的血管生成,有效地缩短了创面闭合时间。LI等[70]证明,脂肪间充质干细胞来源外泌体通过过表达核因子NF-E2相关因子(Nuclear factor-erythroid 2-related factor 2,Nrf2)促进了内皮祖细胞的增殖和血管生成,抑制氧化应激和炎症细胞因子表达来减轻炎症,使得在糖尿病大鼠模型中肉芽组织形成,血管生成增加,生长因子表达水平增加以及炎症和氧化应激相关蛋白水平降低。不仅如此,ZHAO等[71]研究表明,人脂肪间充质干细胞来源外泌体还可以通过负调控基质金属蛋白酶1和基质金属蛋白酶3起到促进胶原合成细胞外基质重塑和伤口愈合的作用。人脂肪间充质干细胞来源外泌体能够调节内皮细胞氧化应激,增强内皮细胞的血管生成以及胶原沉积和细胞外基质重塑,促进糖尿病大鼠伤口愈合[72]。 将外泌体与水凝胶联合使用,似乎也成为了一种可替代治疗创面的高效的新策略。在先前发表的文献中,已经发现脂肪间充质干细胞来源外泌体掺入基于冻融的多肽基水凝胶中具有愈合、抗菌和使外泌体持续释放特性[73]。这些特性有助于通过增强伤口部位的细胞增殖、新生血管形成、再上皮化和胶原重塑来促进伤口愈合[74]。最近,YANG等[75]通过构建PF-127水凝胶和人脐带间充质干细胞来源外泌体联合复合物,并将其应用于糖尿病大鼠皮肤损伤模型中,结果表明与单用人脐带间充质干细胞来源外泌体组、仅使用PF-127对照组相比,联合复合物组显著缩短了糖尿病大鼠模型伤口的愈合时间,CD31和Ki67的表达增加,肉芽组织的再生增强以及血管内皮生长因子和转化生长因子β1表达上调。见表3。 "
| [1] American Diabetes Association. (2) Classification and diagnosis of diabetes. Diabetes Care. 2015;38 Suppl:S8-S16. [2] WILLIAMS R, KARURAGA S, MALAND B, et al. Global and regional estimates and projections of diabetes-related health expenditure: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2020;162:108072. [3] EVERETT E, MATHIOUDAKIS N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci. 2018;1411(1):153-165. [4] RAGHURAM AC, YU RP, LO AY, et al. Role of stem cell therapies in treating Chronic wounds: A systematic review. World J Stem Cells. 2020;12(7):659-675. [5] OBENDORF J, FABIAN C, THOME UH, et al. Paracrine stimulation of perinatal lung functional and structural maturation by mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):525. [6] ZHAO L, HU C, ZHANG P, et al. Preconditioning strategies for improving the survival rate and paracrine ability of mesenchymal stem cells in acute kidney injury. J Cell Mol Med. 2019;23(2):720-730. [7] FRIEDENSTEIN AJ, CHAILAKHYAN RK, LATSINIK NV, et al. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17(4):331-340. [8] KOCHER AA, SCHUSTER MD, SZABOLCS, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7(4):430-436. [9] NAKAGAMI H, MADA K, MORISHITA R, et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005;25(12):2542-2547. [10] CHEN L, TEDGET EE, WUPY, et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3(4):e1886 [11] BRUNO S, GRANGE C, DEREGIBUS, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20(5):1053-1067. [12] SHI Q, QIAN Z, LIU D, et al. GMSC-Derived Exosomes Combined with a Chitosan/Silk Hydrogel Sponge Accelerates Wound Healing in a Diabetic Rat Skin Defect Model. Front Physiol. 2017;8:904. [13] ALTABS V. Diabetes, Endothelial Dysfunction, and Vascular Repair: What Should a Diabetologist Keep His Eye on? . Int J Endocrinol. 2015; 2015:848272. [14] OKONKWO UA, CHEN L, MA D, et al. Compromised angiogenesis and vascular Integrity in impaired diabetic wound healing. PLoS One. 2020; 15(4):e0231962. [15] GUO Y, LIN C, XU P, et al. AGEs Induced Autophagy Impairs Cutaneous Wound Healing via Stimulating Macrophage Polarization to M1 in Diabetes. Sci Rep. 2016;6:36416. [16] AHWA R, GOYAL A, BANSAL P, et al. Chronic Inflammation//StatPearls. FL2021. [17] LOBMANN R, LOUISELLE AE, NIEMIEC SM, et al. Macrophage polarization and diabetic wound healing. Transl Res. 2021;236:109-116. [18] HOSSEINI MANSOUB N. The role of keratinocyte function on the defected diabetic wound healing. Int J Burns Trauma. 2021;11(6):430-441. [19] JHAMB S, VANGAVETI VN, MALABU UH. Genetic and molecular basis of diabetic foot ulcers: Clinical review. Tissue Viability. 2016;25(4):229-236. [20] PASTAR I, STOJADINPVIC O, YIN NC, et al. Epithelialization in Wound Healing: A Comprehensive Review. Adv Wound Care (New Rochelle). 2014;3(7):445-464. [21] HSU EL, STOCK SR. Growth Factors, Carrier Materials, and Bone Repair. Handb Exp Pharmacol. 2020;262:121-156. [22] PARK JW, HWANG SR, YOON IS. Advanced Growth Factor Delivery Systems in Wound Management and Skin Regeneration. Molecules. 2017;22(8):1259. [23] ZUBAIR M, AHMAD J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev Endocr Metab Disord. 2019; 20(2):207-217. [24] OKIZAKI S, LTO Y, HOSONO K, et al. Suppressed recruitment of alternatively activated macrophages reduces TGF-β1 and impairs wound healing in streptozotocin-induced diabetic mice. Biomed Pharmacother. 2015;70:317-325. [25] PATEL S, SRIVATAVA S, SINGH MR, et al. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed Pharmacother. 2019;112:108615. [26] HU C, LI D, PANG Z, et al. Effect of vacuum sealing drainage on expressions of transforming growth factor β1 and its receptor in diabetic foot wound. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018; 32(8):1061-1065. [27] GILLGAN AM, WAYCASTER CR, MOTLEY TA. Cost-effectiveness of becaplermin gel on wound healing of diabetic foot ulcers. Wound Repair Regen. 2015;23(3):353-360. [28] COHEN MA, EAGLSTEIN WH. Recombinant human platelet-derived growth factor gel speeds healing of acute full-thickness punch biopsy wounds. J Am Acad Dermatol. 2001;45(6):857-862. [29] NEVINS M, CAMELO M, NEVINS ML, et al. Periodontal regeneration in humans using recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and allogenic bone. J Periodontol. 2003;74(9):1282-1292. [30] ZHANG J, GUAN M, XIE C, et al. Increased growth factors play a role in wound healing promoted by noninvasive oxygen-ozone therapy in diabetic patients with foot ulcers. Oxid Med Cell Longev. 2014; 2014:273475. [31] OLIVEIRA BC, DE OLIVEIRA BC, DEUTSCH G, et al. Effectiveness of a synthetic human recombinant epidermal growth factor in diabetic patients wound healing: Pilot, double-blind, randomized clinical controlled trial. Wound Repair Regen. 2021;29(6):920-926. [32] FALANGA V,EAGLSTEIN WH, BUCALO B, et al. Topical use of human recombinant epidermal growth factor (h-EGF) in venous ulcers. J Dermatol Surg Oncol. 1992;18(7):604-606. [33] ZHANG JB, LI H, ZHANG L, et al. Observation of curative effect of recombinant human basic fibroblast growth factor combined with compound polymyxin B ointment and local application of insulin on wound healing of deep second-degree burn in diabetes mellitus: a randomized study. Eur Rev Med Pharmacol Sci. 2019;23(13): 5987-5993. [34] MATSUMOTO S, TANAKA R, OKADA K, et al. The Effect of Control-released Basic Fibroblast Growth Factor in Wound Healing: Histological Analyses and Clinical Application. Plast Reconstr Surg Glob Open. 2013; 1(6):e44. [35] ROBSON MC, PHILLIPS TJ, FALANGA V, et al. Randomized trial of topically applied repifermin (recombinant human keratinocyte growth factor-2) to accelerate wound healing in venous ulcers. Wound Repair Regen. 2001;9(5):347-352. [36] LEE CH, LIU KS, CHENG CW, et al. Codelivery of Sustainable Antimicrobial Agents and Platelet-Derived Growth Factor via Biodegradable Nanofibers for Repair of Diabetic Infectious Wounds. ACS Infect Dis. 2020;6(10):2688-2697. [37] AN Y, LIU WJ, XUE P, et al. Autophagy promotes MSC-mediated vascularization in cutaneous wound healing via regulation of VEGF secretion. Cell Death Dis. 2018;9(2):58. [38] OKIZAKI S, LTO Y, HOSONO K, et al. Vascular Endothelial Growth Factor Receptor Type 1 Signaling Prevents Delayed Wound Healing in Diabetes by Attenuating the Production of IL-1β by Recruited Macrophages. Am J Pathol. 2016;186(6):1481-1498. [39] KULWAS A, DRELA E, JUNDZILL, et al. Circulating endothelial progenitor cells and angiogenic factors in diabetes complicated diabetic foot and without foot complications. Diabetes Complications. 2015;29(5):686-690. [40] GANGADARAM P, RAJENDRAN RL,LEE HW, et al. Extracellular vesicles from mesenchymal stem cells activates VEGF receptors and accelerates recovery of hindlimb ischemia. Control Release. 2017;264:112-126. [41] LONG H, LIU D, HE X, et al. A dual functional collagen scaffold coordinates angiogenesis and inflammation for diabetic wound healing. Biomater Sci. 2020;8(22):6337-6349. [42] WHITE MJV, BRIQUEZ PS, WHITE DA, et al.VEGF-A, PDGF-BB and HB-EGF engineered for promiscuous super affinity to the extracellular matrix improve wound healing in a model of type 1 diabetes. NPJ Regen Med. 2021;6(1):76. [43] HEENDRAWAN S, KUSNADI Y, LAGONDA CA, et al. Wound healing potential of human umbilical cord mesenchymal stem cell conditioned medium: An in vitro and in vivo study in diabetes-induced rats. Vet World. 2021;14(8):2109-2117. [44] EI-HATTAB MY, NAGUMO Y, GOURRONC FA, et al. Human Adipocyte Conditioned Medium Promotes In Vitro Fibroblast Conversion to Myofibroblasts. Sci Rep. 2020;10(1):10286. [45] XIN Y, XU P, WANG X, et al.Human foreskin-derived dermal stem/progenitor cell-conditioned medium combined with hyaluronic acid promotes extracellular matrix regeneration in diabetic wounds. Stem Cell Res Ther. 2021;12(1):49. [46] LPK, KANDOI, MISRA R, et al. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019;46:1-9. [47] FUI LW, LOK MPW, GOVINDASAMY V, et al. Understanding the multifaceted mechanisms of diabetic wound healing and therapeutic application of stem cells conditioned medium in the healing process. J Tissue Eng Regen Med. 2019;13(12):2218-2233. [48] GUNAWARDENA TNA, RAHMAN MT, ABDULLAH BJJ, et al. Conditioned media derived from mesenchymal stem cell cultures: The next generation for regenerative medicine. J Tissue Eng Regen Med. 2019;13(4):569-586. [49] HUANG SM, WU CS, CHIU MH, et al. High-glucose environment induced intracellular O-GlcNAc glycosylation and reduced galectin-7 expression in keratinocytes: Implications on impaired diabetic wound healing. J Dermatol Sci. 2017;87(2):168-175. [50] HU SC, LAN CE. High-glucose environment disturbs the physiologic functions of keratinocytes: Focusing on diabetic wound healing. J Dermatol Sci. 2016;84(2):121-127. [51] SAHELI M, BAYAT M, GANJI R, et al. Human mesenchymal stem cells-conditioned medium improves diabetic wound healing mainly through modulating fibroblast behaviors. Arch Dermatol Res. 2020;312(5):325-336. [52] LUO H, WANG Y, SU Y, et al. Paracrine effects of adipose-derived stem cells in cutaneous wound healing in streptozotocin-induced diabetic rats. J Wound Care. 2022;31:S29-S38. [53] JOSEPH A, BAIJU I,BHAT IA, et al. Mesenchymal stem cell-conditioned media: A novel alternative of stem cell therapy for quality wound healing. Cell Physiol. 2020;235(7-8):5555-5569. [54] TAKAHASHI H, OHNISHI S,YAMAOTO Y, et al. Topical Application of Conditioned Medium from Hypoxically Cultured Amnion-Derived Mesenchymal Stem Cells Promotes Wound Healing in Diabetic Mice. Plast Reconstr Surg. 2021;147(6):1342-1352. [55] VEITH AP, HENDER SON K, SPENCER A, et al.Therapeutic strategies for enhancing angiogenesis in wound healing. Adv Drug Deliv Rev. 2019;146:97-125. [56] ZHANG S, CHENL, ZHANG G, et al. Umbilical cord-matrix stem cells induce the functional restoration of vascular endothelial cells and enhance skin wound healing in diabetic mice via the polarized macrophages. Stem Cell Res Ther. 2020;11(1):39. [57] YAMAGUCHI J, KUSANO KF, MASUO O, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107(9): 1322-1328. [58] GALLAGHER KA, LIU ZJ, XIAO M, et al. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. Clin Invest. 2007;117(5):1249-1259. [59] FRIDONI M, KOUHKHEIL R, ABDLLHIFAR MA, et al. Improvement in infected wound healing in type 1 diabetic rat by the synergistic effect of photobiomodulation therapy and conditioned medium. Cell Biochem. 2019;120(6):9906-9916. [60] HU CH, TSENG YW,CHIOU CY, et al. Bone marrow concentrate-induced mesenchymal stem cell conditioned medium facilitates wound healing and prevents hypertrophic scar formation in a rabbit ear model. Stem Cell Res Ther. 2019;10(1):275. [61] KOUREMBANAS S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy . Annu Rev Physiol. 2015;77: 13-27. [62] CORRADO C, RAIMONO S, CHIESI A, et al. Exosomes as intercellular signaling organelles involved in health and disease: basic science and clinical applications. Int J Mol Sci. 2013;14(3):5338-5366. [63] HYLDIG K, RIIS S, PENNISI CP, et al. Implications of Extracellular Matrix Production by Adipose Tissue-Derived Stem Cells for Development of Wound Healing Therapies. Int J Mol Sci. 2017;18(6):1167. [64] HAN G, CEILLEY R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv Ther. 2017;34(3):599-610. [65] THAN UTT, GUANZON D, LEAVESLEYD, et al. Association of Extracellular Membrane Vesicles with Cutaneous Wound Healing. Int J Mol Sci. 2017;18(5):956. [66] SCHWAB A, MEYERING SS, LEPENE B, et al. Extracellular vesicles from infected cells: potential for direct pathogenesis. Front Microbiol. 2015; 6:1132. [67] HUANG C, LUO W, WANG Q, et al. Human mesenchymal stem cells promote ischemic repairment and angiogenesis of diabetic foot through exosome miRNA-21-5p. Stem Cell Res. 2021;52:102235. [68] LI X, LIU L, YANG J, et al. Exosome Derived From Human Umbilical Cord Mesenchymal Stem Cell Mediates MiR-181c Attenuating Burn-induced Excessive Inflammation. EBio Medicine. 2016;8:72-82. [69] YU M, LIU W, LI J, et al. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther. 2020;11(1):350. [70] LI X, XIE X, LIAN W, et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018; 50(4):1-14. [71] ZHAO B, ZHANG X,ZHANG Y, et al. Human Exosomes Accelerate Cutaneous Wound Healing by Promoting Collagen Synthesis in a Diabetic Mouse Model. Stem Cells Dev. 2021;30(18):922-933. [72] YAN C, XV Y , LIN Z, et al. Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Accelerate Diabetic Wound Healing via Ameliorating Oxidative Stress and Promoting Angiogenesis. Front Bioeng Biotechnol. 2022;10:829868. [73] SHEN YI, CHO H, PAPA AE,et al. Engineered human vascularized constructs accelerate diabetic wound healing. Biomaterials. 2016;102: 107-119. [74] WANG C, WANG M, XU T, et al. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics. 2019; 9(1):65-76. [75] YANG J,CHEN Z,PAN D, et alUmbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomes Combined Pluronic F127 Hydrogel Promote Chronic Diabetic Wound Healing and Complete Skin Regeneration. Int J Nanomedicine. 2020;15:5911-5926. |
| [1] | Pan Zhongjie, Qin Zhihong, Zheng Tiejun, Ding Xiaofei, Liao Shijie. Targeting of non-coding RNAs in the pathogenesis of the osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1441-1447. |
| [2] | Dang Yi, Du Chengyan, Yao Honglin, Yuan Nenghua, Cao Jin, Xiong Shan, Zhang Dingmei, Wang Xin. Hormonal osteonecrosis and oxidative stress [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1469-1476. |
| [3] | Yang Zhishan, Tang Zhenglong. YAP/TAZ, a core factor of the Hippo signaling pathway, is involved in bone formation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1264-1271. |
| [4] | Xu Cong, Zhao He, Sun Yan. Regeneration of facial nerve injury repaired by biomaterial nerve conduits [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1089-1095. |
| [5] | Tang Haotian, Liao Rongdong, Tian Jing. Application and design of piezoelectric materials for bone defect repair [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1117-1125. |
| [6] | Xue Ting, Zhang Xinri, Kong Xiaomei. Mesenchymal stem cell therapy for pneumoconiosis using nanomaterials combined with multi-modal molecular imaging [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1133-1140. |
| [7] | Liu Wentao, Feng Xingchao, Yang Yi, Bai Shengbin. Effect of M2 macrophage-derived exosomes on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 840-845. |
| [8] | Long Yanming, Xie Mengsheng, Huang Jiajie, Xue Wenli, Rong Hui, Li Xiaojie. Casein kinase 2-interaction protein-1 regulates the osteogenic ability of bone marrow mesenchymal stem cells in osteoporosis rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 878-882. |
| [9] | Cui Lianxu, Jiang Wenkang, Lu Dahong, Xu Junrong, Liu Xiaocui, Wang Bingyun. Clinical-grade human umbilical cord mesenchymal stem cells affect the improvement of neurological function in rats with traumatic brain injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 835-839. |
| [10] | Li Qicheng, Deng Jin, Fu Xiaoyang, Han Na. Effects of bone marrow mesenchymal stem cells-derived exosomes on hypoxia-treated myoblasts [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 853-859. |
| [11] | Wang Min, Yin Xiushan, Wang Yingxi, Zhang Yan, Zhao Long, Xia Shuyue. Inhalation of bone marrow mesenchymal stem cells-derived exosomes alleviates inflammatory injury in chronic obstructive pulmonary disease [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 827-834. |
| [12] | Zhang Houjun, Deng Bowen, Jiang Shengyuan, Zhao Yi, Ren Jingpei, Xu Lin, Mu Xiaohong. Proteomic analysis of cerebrospinal fluid exosomes derived from cerebral palsy children [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 903-908. |
| [13] | Gao Ting, Ma Xiaohong, Li Xiaorong. Extraction and identification of exosomes from three different sources of ovarian granulosa cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 860-865. |
| [14] | Ke Weiqiang, Chen Xianghui, Chen Xiaoling, Meng Jie, Ma Yanlin. Rituximab combined with autologous peripheral blood stem cell transplantation in the treatment of diffuse large B-cell lymphoma and the expression of related factors [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 915-920. |
| [15] | Huang Guijiang, Ji Yuwei, Zhao Xin, Yang Yi, Zhao Yulan, Wang Peijin, Tang Wei, Jiao Jianlin. Effect and mechanism of different administration routes of placenta-derived mesenchymal stem cells in the treatment of tree shrews with osteoporotic fracture [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 909-914. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||