Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (9): 1441-1447.doi: 10.12307/2023.227
Previous Articles Next Articles
Targeting of non-coding RNAs in the pathogenesis of the osteonecrosis of the femoral head
Pan Zhongjie1, Qin Zhihong1, Zheng Tiejun2, Ding Xiaofei1, Liao Shijie1
- 1Departments of Orthopedics and Hand Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning 530022, Guangxi Zhuang Autonomous Region, China; 2Collaborative Innovation Center of Regenerative Medicine and Medical Biological Resources Development and Application, Guangxi Medical University, Nanning 530022, Guangxi Zhuang Autonomous Region, China
-
Received:2021-12-01Accepted:2022-03-12Online:2023-03-28Published:2022-07-02 -
Contact:Liao Shijie, MD, Associate chief physician, Departments of Orthopedics and Hand Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning 530022, Guangxi Zhuang Autonomous Region, China -
About author:Pan Zhongjie, Master candidate, Departments of Orthopedics and Hand Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning 530022, Guangxi Zhuang Autonomous Region, China -
Supported by:the National Natural Science Foundation of China, No. 82060396 (to DXF); General Project of Guangxi Natural Science Foundation, No. 2017GXNSFAA198305 (to DXF); the National Natural Science Foundation of China, No. 82160809 (to LSJ); Youth Fund Project of Guangxi Natural Science Foundation, No. 2018GXNSFBA281090 (to LSJ)
CLC Number:
Cite this article
Pan Zhongjie, Qin Zhihong, Zheng Tiejun, Ding Xiaofei, Liao Shijie. Targeting of non-coding RNAs in the pathogenesis of the osteonecrosis of the femoral head[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1441-1447.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
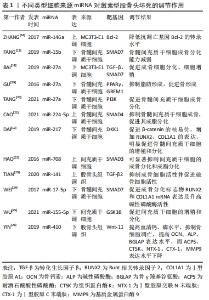
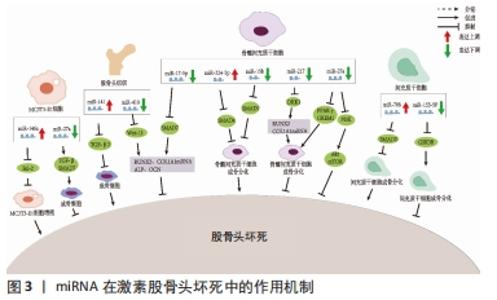
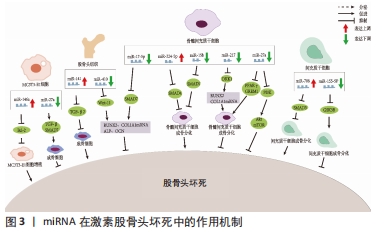
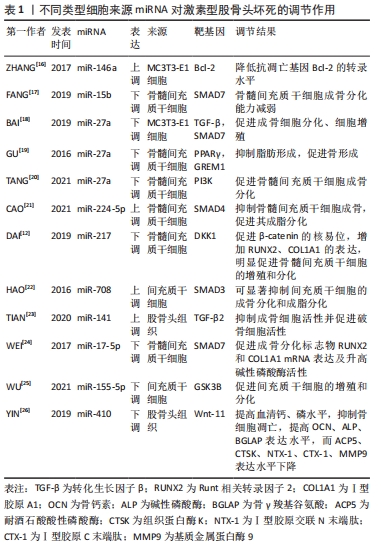
2.1 非编码RNA的概述 在人类的基因组中只有1.5%是蛋白质编码区,而其余没有蛋白质编码能力的核苷酸序列统称为非编码RNA [7]。非编码RNA最初被认为是无用的RNA,但随着高通量测序技术的进展, 非编码RNA现在被广泛认为是基因表达的重要调控因子,在转录时和转录后都能发挥作用。尽管在位置、结构、长度和功能上存在差异,但非编码RNA大约占所有转录产物的98%,这些非编码RNA在正常基因表达和疾病进展中发挥作用,使其成为机制研究和治疗潜力的新靶点[4]。非编码RNA可占所有RNA的90%左右,根据其功能可分为管家非编码RNA,如tRNA、rRNA、snoRNA、snRNA等,以及调节非编码RNA,如miRNA、lncRNA、circRNA、siRNA等[8]。随着非编码RNA 研究的深入,其不仅仅可以调控基因的表达、还可以在细胞间信息传递和作为潜在的疾病诊断标记物。目前所研究的非编码RNA大多是miRNA、lncRNA和circRNA,因此该综述主要关注miRNAs、lncRNAs和circRNAs与非创伤性股骨头坏死的研究进展。 2.2 miRNAs与股骨头坏死 miRNAs是一组小片段、单链、内源性非编码RNA,主要通过与靶mRNA的3 ‘-非翻译区(UTR)结合来调节其翻译或稳定性,从而负调控靶mRNA的表达。先前的研究表明,miRNAs在多种生理过程中发挥重要作用,包括细胞发育、增殖、分化、代谢、迁移和凋亡[9]。 目前糖皮质激素诱导的股骨头坏死占非创伤性股骨头坏死病因的主要部分。糖皮质激素诱导的股骨头坏死具有复杂的病理过程,多种内外因素导致髓内微血管病变,血栓形成导致股骨头供血、供氧不足,骨细胞死亡[10]。然而,确切发病机制和分子机制仍不清楚。由于在临床上不能通过活组织检查来诊断早期激素型股骨头坏死,因此需要找到一种用于早期疾病诊断的生物化学标志物。2017年LI等[11]纳入3例激素型股骨头坏死患者,其股骨头坏死塌陷区与非塌陷区的组织细胞RNA进行比较,发现有一组miRNA的差异表达超过2倍,具体来说,有8个miRNA上调(hsa-miR-4472,hsa-miR-4306,hsa-miR-4747-5p,hsa-miR-4441,hsa-miR-4709-3p,ebv-miR-BHRF1-2-3p,hsa-miR-585-3p和hsa-miR-5572),2个miRNA下调(hsa-miR-195-5p和hsa-miR-645)。DAI等[12]通过对10例激素型股骨头坏死患者和10例股骨颈骨折患者的股骨近端骨髓的骨髓间充质干细胞进行miRNA测序,发现股骨头坏死患者的样本中miR-217的表达水平显著降低,miR-217通过抑制DKK1的表达,促进了β-catenin的核易位,增加了成骨标记物RUNX2、COL1A1的表达,并明显促进了骨髓间充质干细胞的增殖和分化,恢复骨髓间充质干细胞中DKK1的表达部分逆转了miR-217的作用。这些结果表明,miR-217在激素相关骨坏死的发生过程中通过抑制DKK1的表达促进骨髓间充质干细胞的增殖和成骨分化,从而阻止股骨头坏死的进展。 目前miRNA与股骨头坏死的研究流程大多是:①通过高通量测序等方法筛选出导致疾病的可能基因;②通过生信分析、双荧光素酶实验和转染后的靶蛋白表达水平检测确定miRNA 分子在激素型股骨头坏死中发挥调控作用的靶点。研究人员常利用miRNA类似物和抑制剂在细胞中验证目标miRNA在激素型股骨头坏死发病过程中的作用,在体内实验中,使用miRNA分子或其转染的细胞进行动物模型的干预[13-15]。目前文献中所报道的不同类型细胞来源的miRNA对激素型股骨头坏死的调节作用,见表1[12,16-26],作用机制,见图3。 "
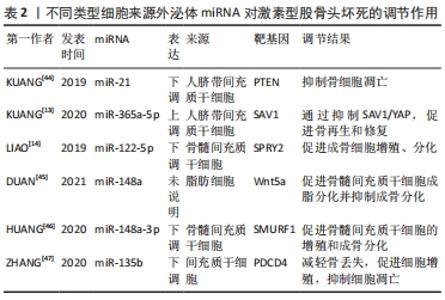
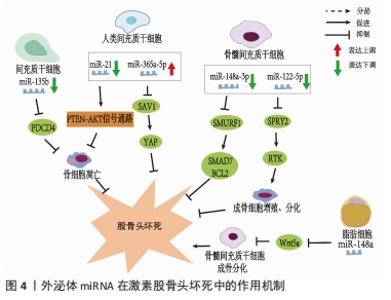
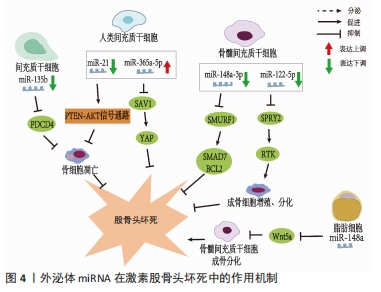
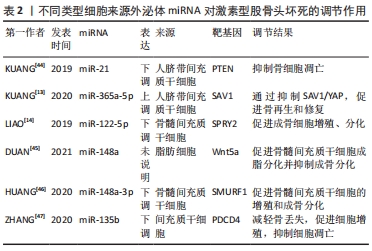
值得注意的是,近年来随着外泌体研究的深入,来源于外泌体的非编码RNA在非创伤性股骨头坏死中的作用机制越来越清晰。据报道非编码RNA在外泌体中更稳定[27],因此目前外泌体所携带的非编码RNA在非创伤性股骨头坏死中的研究也逐渐成为热点。外泌体特指直径40-100 nm的细胞外囊泡,囊泡内含有DNA、mRNA、microRNA、脂类和蛋白质等,通过其所携带泡内物质的转移实现细胞间的内部信息相互传递,在细胞间通讯中起重要作用[28]。外泌体从一开始的细胞“废物”逐渐转变为在生理病理中都具有重要意义的物质,其主要功能不仅与内容物有关,还与其表面蛋白有关。同时外泌体包含胞质和核蛋白、膜蛋白、细胞外基质蛋白、核酸和代谢物,即DNA、mRNA和非编码RNA,其所含内容的丰富性,提示其功能的多样性[29-30]。它被发现在细胞间通讯、炎症反应、免疫反应、神经退行性疾病、代谢和心血管疾病、肿瘤等相关疾病中都具有重要作用[31]。在目前的研究中,外泌体可以在多种细胞外液体中发现,如血液、唾液、脑脊液、羊水、母乳[32]、尿液、精液[33]、脑脊液、肺泡灌洗液和恶性腹水中[34],最近研究发现关节液中也含有外泌体[35]。ZHANG等[36]发现间充质干细胞来源外泌体和包含siRNAs的外泌体能有效促进股骨头坏死中的血管再生,加快其修复,为股骨头坏死患者带来新的希望。 目前的研究表明外泌体富含非编码RNA,包括miRNAs、lncRNAs、circRNA、Piwi相互作用 RNA (piRNAs) 和 tRNA 衍生的小非编码 RNA(TsRNA)[37]。与细胞内RNA不同,由于它们有双层膜结构和能结合RNA的蛋白,以及外泌体阻止其作为载体的非编码RNA在细胞外被核糖核酸酶降解,因此外泌体非编码RNA更稳定,从而使外泌体非编码RNA的临床应用成为可能[27]。研究人员已经发现外泌体非编码RNA可以被转移到受体细胞,并在细胞外基质形成、凋亡、增殖、迁移和炎症中发挥作用[38]。目前外泌体非编码RNA与股骨头坏死研究中集中在miRNAs上,lncRNAs、circRNAs与股骨头坏死的相关研究尚未见到报道,其具体原因尚未清楚,而外泌体lncRNAs、circRNAs在其他疾病中研究较多,如心血管系统疾病[39]、泌尿系统肿瘤[40]、消化系统肿瘤[41]、呼吸系统肿瘤[39],并且大多数可作为诊断的生物化学标志物。因此未来阐明外泌体特定lncRNAs、circRNAs与非创伤性股骨头坏死的发病机制和治疗有着巨大的前景。 VALADI等[42]研究中首次发现并初步证实了外泌体可以同时包含mRNA和miRNA,称为“外泌体穿梭RNA”(esRNA),它们可以被转移到新的细胞,并翻译成蛋白质发挥功能。这一发现改变了目前人们对外泌体的传统看法,认为外泌体可以有效调节受体细胞基因的表达水平。ZHANG等[43]鉴定了5例无激素型股骨头坏死的系统性红斑狼疮患者、5例有激素型股骨头坏死的系统性红斑狼疮患者和5例健康者的新鲜血清中获得的外泌体的特征,综合外泌体miRNA测序以分析3组中差异表达的miRNA,然后通过qRT-PCR验证所选miRNA的表达水平,构建KEGG通路、GO分析、蛋白-蛋白相互作用(PPI)网络和miRNAs-mRNA相互作用网络,分析其潜在靶点和机制。测序数据显示,hsa-miR-135b-5p、hsa-miR-150-5p、hsa-miR-509-3-5p、hsa-miR-514a-3p和hsa-miR-708-5p在3组中均有显著差异表达,并进一步提出hsa-miR-135b-5p可能被作为激素型股骨头坏死的唯一诊断生物标志物。 目前文献中所报道的不同类型细胞来源的外泌体miRNA对激素型股骨头坏死的调节作用,见表2[13-14,44-47],作用机制,见图4。 "
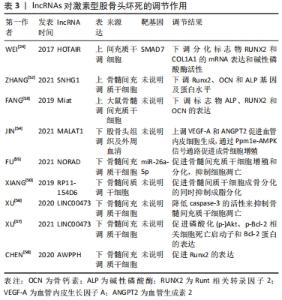
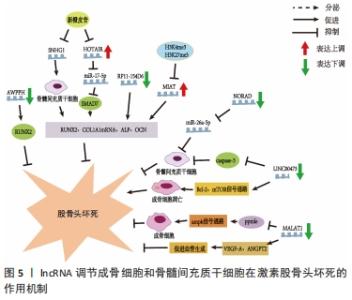
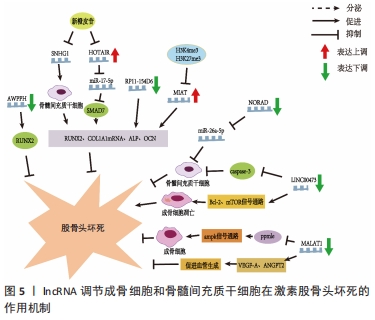
此外,在创伤性股骨头坏死方面,XU等[15]的研究指出了骨髓间充质干细胞所携带miR-224-3p的外泌体在创伤性股骨头坏死中的作用,结果表现miR-224-3p在创伤性股骨头坏死中下调,FIP200被证实为miR-224-3p的靶基因,在体内miR-224-3p的下调会上调FIP200而有效促进血管内皮细胞增殖、迁移和侵袭,从而阻止股骨头坏死进展。 2.3 lncRNAs与股骨头坏死 真核生物中的非编码RNA种类繁多,多于蛋白质编码基因的数量。除了不同家族的小非编码RNA外,大部分转录组产生的RNA转录量都超过了200个核苷酸,这些RNA通常是聚腺苷化的,并且缺乏明显的开放阅读框(ORFs),这些被定义为lncRNAs[48]。目前的研究中发现lncRNAs在多种生理和病理变化过程中可以发挥重要作用,包括细胞增殖、细胞死亡、干细胞多能性、代谢控制、衰老和变性,以及炎症和免疫反应[48]。最近研究也表明,lncRNAs可调控骨发育和再生,并参与不同骨科疾病的发病机制[49],如骨关节炎、骨质疏松、骨肉瘤和椎间盘退变。最近,lncRNAs也被发现参与了股骨头坏死的发展[6],利用反转录-定量PCR技术鉴定出股骨头坏死骨组织[45]、间充质干细胞和骨微血管内皮细胞中lncRNA表达的差异[50]。LI等[51]首次描述了人骨髓源性间充质干细胞成骨分化过程中特异性lncRNAs在股骨头坏死成骨分化中的作用,他们利用芯片分析和RT-qPCR对人骨髓源性间充质干细胞成骨分化过程中的lncRNAs表达谱进行了分析,以验证芯片数据,共鉴定出24个下调和24个上调的lncRNA,RT-qPCR结果与芯片分析结果一致;利用GO、KEGG数据库进行生物信息学分析,以探索可能的机制和识别lncRNA参与的信号通路;GO分析结果显示细胞内细胞器、Ras蛋白信号转导和转移酶活性发生显著变化;KEGG通路分析显示,lncRNAs与脂肪酸代谢、细胞凋亡及转化生长因子信号通路密切相关;MAPT反义RNA 1(MAPT AS1)的过表达在细胞和mRNA水平上促进人骨髓源性间充质干细胞成骨和抑制脂肪形成。另有文献报道了外泌体lncRNA与激素型股骨头坏死的关系,其调节作用见表3[24,50,52-58],作用机制,见图5。 "
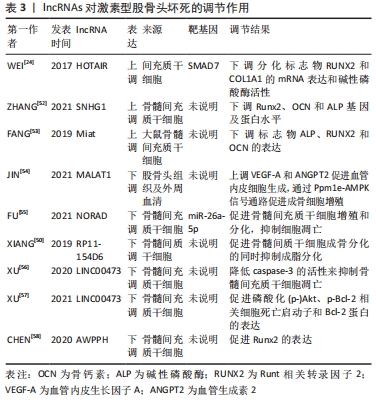

2.3.1 lncRNAs与成骨分化、增殖的关系 WEI等[24]采用实时荧光定量PCR检测非创伤性股骨头坏死患者间充质干细胞中HOTAIR和miR-17-5p的表达水平,采用骨形态发生蛋白2诱导人骨髓间充质干细胞进行成骨分化,结果与骨关节炎相比,非创伤性股骨头坏死患者miR-17-5p表达水平较低,HOTAIR表达水平较高,si-HOTAIR诱导HOTAIR下调导致miR-17-5p表达增加,miR-17-5p靶基因SMAD7表达降低,成骨分化标志物RUNX2、COL1A1的mRNA表达和碱性磷酸酶活性也在si-HOTAIR作用下升高。然而,miR-17-5p抑制剂或SMAD7上调后,这些值的增加被抵消,表明HOTAIR可以通过调节miR-17-5p及其靶基因SMAD7在非创伤性股骨头坏死中发挥调控成骨分化和增殖的作用。YUAN等[59]发现HOTAIR在骨髓间充质干细胞成骨分化后表达下调,成脂分化后表达上调。HOTAIR过表达抑制骨髓间充质干细胞成骨分化及RUNX2、OCN、ALP的表达,而增加成脂分化及LPL、PPARγ的表达。研究结果表明新橙皮苷通过抑制lncRNA SNHG1的组蛋白修饰提高人骨髓间充质干细胞的成骨分化能力、碱性磷酸酶活性以及RUNX2、OCN和ALP的表达[52]。最近的一项研究显示,与激素型股骨头坏死患者的非坏死组织相比,骨坏死组织中的lncRNA Miat水平显著升高;通过设计大鼠Miat的siRNAs来沉默大鼠骨髓间充质干细胞中Miat的内源性表达,两种siRNAs(siMiat-1和-2)对Miat有显著的抑制作用,结果表明成骨标志物ALP、RUNX2和OCN的表达升高[53]。活血通络胶囊可以通过改变组蛋白修饰H3K4me3和H3K27me3来显著抑制rMiat的表达[53]。这些结果表明,活血通络胶囊可能通过对Miat的表达沉默来抑制股骨头坏死的发展。 2.3.2 lncRNAs与血管内皮细胞的关系 骨微血管内皮细胞主要由mRNA和lncRNA组成,其转录组的适当表达和调控对包括骨髓间充质干细胞生理活动在内的所有生物学和细胞过程都至关重要。在经过糖皮质激素干预后,相对于对照组有73个lncRNA表达上调,166个lncRNA表达下调,其中107个lncRNA的表达与氢化可的松诱导的172个mRNA表达显著相关[60]。在激素型股骨头坏死患者中,LncRNA MALAT1在骨髓间充质干细胞成骨分化过程中显著下调;此外,MALAT1通过激活Ppm1e-AMPK信号通路对激素诱导的成骨细胞具有一定保护作用,而在其他条件下,MALAT1也被证实具有促进血管生成的作用;例如MALAT1可以通过上调VEGF-A和ANGPT2促进脑微血管内皮细胞的血管生成;通过诱导血管内皮生长因子促进间充质干细胞的血管生成[54]。JIN等[54]研究发现非创伤性股骨头坏死患者血清中LncRNA MALAT1表达明显低于健康对照组,进一步的ROC曲线分析表明,血清MALAT1可作为诊断非创伤性股骨头坏死的良好指标。 2.3.3 lncRNAs与骨髓间充质干细胞的关系 骨髓间充质干细胞是一种起源于中胚层的成体干细胞,具有自我更新和多向分化潜能,在机体中具有重要作用,骨髓间充质干细胞可分化为成骨细胞、成软骨细胞和脂肪细胞,进而分泌生长因子促进组织再生[61],越来越多的证据表明lncRNAs参与调控骨髓间充质干细胞凋亡[62]。 (1) LncRNA NORAD:是一种被DNA损伤激活的长链非编码RNA,在多种肿瘤中异常表达,是一种癌基因。FU等[55]发现激素型股骨头坏死组织中NORAD表达下调,miR-26a-5p表达上调。过表达NORAD可改善地塞米松诱导的骨髓间充质干细胞增殖和分化抑制,促进细胞凋亡,而下调NORAD则会导致相反的结果。此外,NORAD通过调控miR-26a-5p改善地塞米松诱导的骨髓间充质干细胞增殖和分化的抑制,并促进细胞凋亡。 (2) lncRNA RP11-154D6:在股骨头坏死患者骨髓间质干细胞中的表达明显降低,证实了lncRNA RP11-154D6在促进骨髓间质干细胞成骨分化的同时抑制成脂分化[50]。 (3) LINC00473:是一个基因间lncRNA,由2个外显子组成,位于人类染色体6q27上[63]。最近的研究发现LINC00473在激素型股骨头坏死患者的骨髓间充质干细胞中会显著下调[57]。同时通过FISH实验确定了LINC00473在骨髓间充质干细胞中的亚细胞定位,发现LINC00473同时位于骨髓间充质干细胞的细胞质和细胞核中;上调LINC00473显著降低了地塞米松诱导的骨髓间充质干细胞凋亡,并且LINC00473的抗凋亡作用持续到第7天;在浓度为10-6 mol/L的情况下可以增加caspase-3的活性,而LINC00473的上调减弱了这种作用,说明LINC00473可以通过降低caspase-3活性来抑制地塞米松诱导的骨髓间充质干细胞凋亡[56]。此外,有报道称地塞米松可通过上调Bcl-2表达和激活mTOR信号通路诱导成骨细胞凋亡[64]。在他们随后的研究中发现上调LINC00473减弱了1 μmol/L地塞米松对骨髓间充质干细胞增殖和凋亡的抑制作用;此外,上调LINC00473可显著促进磷酸化Akt、Bad和Bcl-2蛋白的表达,但降低了caspase-3的裂解,从而抑制了地塞米松诱导的人骨髓间充质干细胞凋亡[57]。 (4) LncRNA AWPPH:与健康对照组相比,非创伤性股骨头坏死患者的骨髓间质干细胞和血清中AWPPH均显著下调,与患者年龄、性别、生活习惯无显著相关性,但与病程有显著相关性,因此AWPPH在间充质干细胞和血清中的表达是非创伤性股骨头坏死的敏感诊断标志物;在骨髓间充质干细胞中,AWPPH过表达促进了Runx2表达,而AWPPH短发夹RNA沉默抑制了Runx2的表达[58]。因此,可以推断lncRNA AWPPH可能通过上调Runx2参与股骨头坏死的发生发展。 2.4 circRNAs与股骨头坏死 与其他类型的非编码RNA相比,关于circRNAs在类固醇诱导的股骨头坏死中的研究仍处于较早期的阶段。KUANG等[65]报道了激素诱导的股骨头坏死中circUSP45与骨代谢的关系,为了验证circUSP45在体内的功能,作者使用了类固醇诱导的股骨头坏死大鼠模型,发现circUSP45通过吞噬miR-127-5p和靶向PTEN/AKT通路调控骨髓间充质干细胞的成骨和增殖,而抑制circUSP45可以增加骨量。在相同通路上,circ_0066523通过表观遗传学抑制PTEN从而激活AKT通路促进骨髓间充质干细胞增殖、分化[66]。 JIANG等[67]报道了非创伤性股骨头坏死患者血浆circ CDR1as表达明显增高,局部坏死组织中的circCDR1as明显高于相邻的未受累组织,影像学分期ARCO4期患者的血浆和局部circCDR1as表达明显高于ARCO3期患者;与ARCO1/2q期相比,ARCO3期患者血浆和局部circCDR1as表达显著上调。血浆和局部circCDR1as表达与ARCO分类呈正相关;此外,血浆和局部circCDR1as表达与疾病严重程度呈正相关。 "

| [1] MA J, HUA XY, ZHENG MX, et al. Surface-based map plasticity of brain regions related to sensory motor and pain information processing after osteonecrosis of the femoral head. Neural Regen Res. 2022;17(4):806-811. [2] 中国医师协会骨科医师分会骨循环与骨坏死专业委员会,中华医学会骨科分会骨显微修复学组,国际骨循环学会中国区.中国成人股骨头坏死临床诊疗指南(2020)[J].中华骨科杂志,2020,40(20):1365-1376. [3] XIE K, MAO Y, QU X, et al. High-energy extracorporeal shock wave therapy for nontraumatic osteonecrosis of the femoral head. J Orthop Surg Res. 2018;13(1):25. [4] BEERMANN J, PICCOLI MT, VIERECK J, et al. Non-coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol Rev. 2016;96(4):1297-1325. [5] HASHEMIAN SM, POURHANIFEH MH, FADAEI S, et al. Non-coding RNAs and Exosomes: Their Role in the Pathogenesis of Sepsis. Mol Ther Nucleic Acids. 2020;21:51-74. [6] LI Z, HUANG C, YANG B, et al. Emerging roles of long non-coding RNAs in osteonecrosis of the femoral head. Am J Transl Res. 2020;12(9):5984-5991. [7] LANDER ES. Initial impact of the sequencing of the human genome. Nature. 2011;470(7333):187-197. [8] 李崇,缪季峰,林秋宁,等.外泌体非编码RNA在骨关节炎软骨损伤修复中的研究进展[J].中华骨科杂志,2021,41(3):186-194. [9] SUN Q, YANG Z, LI P, et al. A novel miRNA identified in GRSF1 complex drives the metastasis via the PIK3R3/AKT/NF-κB and TIMP3/MMP9 pathways in cervical cancer cells. Cell Death Dis. 2019;10(9):636. [10] CHANG C, GREENSPAN A, GERSHWIN ME. The pathogenesis, diagnosis and clinical manifestations of steroid-induced osteonecrosis. J Autoimmun. 2020;110:102460. [11] LI P, ZHAI P, YE Z, et al. Differential expression of miR-195-5p in collapse of steroid-induced osteonecrosis of the femoral head. Oncotarget. 2017;8(26): 42638-42647. [12] DAI Z, JIN Y, ZHENG J, et al. MiR-217 promotes cell proliferation and osteogenic differentiation of BMSCs by targeting DKK1 in steroid-associated osteonecrosis. Biomed Pharmacother. 2019;109:1112-1119. [13] KUANG MJ, ZHANG KH, QIU J, et al. Exosomal miR-365a-5p derived from HUC-MSCs regulates osteogenesis in GIONFH through the Hippo signaling pathway. Mol Ther Nucleic Acids. 2020;23:565-576. [14] LIAO W, NING Y, XU HJ, et al. BMSC-derived exosomes carrying microRNA-122-5p promote proliferation of osteoblasts in osteonecrosis of the femoral head. Clin Sci (Lond). 2019;133(18):1955-1975. [15] XU HJ, LIAO W, LIU XZ, et al. Down-regulation of exosomal microRNA-224-3p derived from bone marrow-derived mesenchymal stem cells potentiates angiogenesis in traumatic osteonecrosis of the femoral head. FASEB J. 2019; 33(7):8055-8068. [16] ZHANG B, YI J, ZHANG CL, et al. MiR-146a inhibits proliferation and induces apoptosis in murine osteoblastic MC3T3-E1 by regulating Bcl2. Eur Rev Med Pharmacol Sci. 2017;21(17):3754-3762. [17] FANG SH, CHEN L, CHEN HH, et al. MiR-15b ameliorates SONFH by targeting Smad7 and inhibiting osteogenic differentiation of BMSCs. Eur Rev Med Pharmacol Sci. 2019;23(22):9761-9771. [18] BAI Y, LIU Y, JIN S, et al. Expression of microRNA‑27a in a rat model of osteonecrosis of the femoral head and its association with TGF‑β/Smad7 signalling in osteoblasts. Int J Mol Med. 2019;43(2):850-860. [19] GU C, XU Y, ZHANG S, et al. miR-27a attenuates adipogenesis and promotes osteogenesis in steroid-induced rat BMSCs by targeting PPARγ and GREM1. Sci Rep. 2016;6:38491. [20] TANG J, YU H, WANG Y, et al. miR-27a promotes osteogenic differentiation in glucocorticoid-treated human bone marrow mesenchymal stem cells by targeting PI3K. J Mol Histol. 2021;52(2):279-288. [21] CAO Y, JIANG C, WANG X, et al. Reciprocal effect of microRNA-224 on osteogenesis and adipogenesis in steroid-induced osteonecrosis of the femoral head. Bone. 2021;145:115844. [22] HAO C, YANG S, XU W, et al. MiR-708 promotes steroid-induced osteonecrosis of femoral head, suppresses osteogenic differentiation by targeting SMAD3. Sci Rep. 2016;6:22599. [23] TIAN L, SUN S, LI W, et al. Down-regulated microRNA-141 facilitates osteoblast activity and inhibits osteoclast activity to ameliorate osteonecrosis of the femoral head via up-regulating TGF-β2. Cell Cycle. 2020;19(7):772-786. [24] WEI B, WEI W, ZHAO B, et al. Long non-coding RNA HOTAIR inhibits miR-17-5p to regulate osteogenic differentiation and proliferation in non-traumatic osteonecrosis of femoral head. PLoS One. 2017;12(2):e0169097. [25] WU F, HUANG W, YANG Y, et al. miR-155-5p regulates mesenchymal stem cell osteogenesis and proliferation by targeting GSK3B in steroid-associated osteonecrosis. Cell Biol Int. 2021;45(1):83-91. [26] YIN Y, DING L, HOU Y, et al. Upregulating MicroRNA-410 or Downregulating Wnt-11 Increases Osteoblasts and Reduces Osteoclasts to Alleviate Osteonecrosis of the Femoral Head. Nanoscale Res Lett. 2019;14(1):383. [27] MIAO C, ZHOU W, WANG X, et al. The Research Progress of Exosomes in Osteoarthritis, With Particular Emphasis on the Mediating Roles of miRNAs and lncRNAs. Front Pharmacol. 2021;12:685623. [28] MATHIEU M, MARTIN-JAULAR L, LAVIEU G, et al. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9-17. [29] PATHAN M, FONSEKA P, CHITTI SV, et al. Vesiclepedia 2019: a compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2019;47(D1):D516-D519. [30] VAN BALKOM BW, EISELE AS, PEGTEL DM, et al. Quantitative and qualitative analysis of small RNAs in human endothelial cells and exosomes provides insights into localized RNA processing, degradation and sorting. J Extracell Vesicles. 2015;4:26760. [31] KALLURI R, LEBLEU VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. [32] HE C, ZHENG S, LUO Y, et al. Exosome Theranostics: Biology and Translational Medicine. Theranostics. 2018;8(1):237-255. [33] SULLIVAN R, SAEZ F, GIROUARD J, et al. Role of exosomes in sperm maturation during the transit along the male reproductive tract. Blood Cells Mol Dis. 2005;35(1):1-10. [34] LUDWIG AK, GIEBEL B. Exosomes: small vesicles participating in intercellular communication. Int J Biochem Cell Biol. 2012;44(1):11-15. [35] ASGHAR S, LITHERLAND GJ, LOCKHART JC, et al. Exosomes in intercellular communication and implications for osteoarthritis. Rheumatology (Oxford). 2020;59(1):57-68. [36] ZHANG C, SU Y, DING H, et al. Mesenchymal stem cells-derived and siRNAs-encapsulated exosomes inhibit osteonecrosis of the femoral head. J Cell Mol Med. 2020;24(17):9605-9612. [37] 刘超,曾昭穆,温稀超,等.外泌体非编码RNA在胶质瘤发生发展中的作用及其临床应用前景[J].中国组织工程研究,2022,26(24):3928-3936. [38] CHEN J, YU X, ZHANG X. Advances on biological functions of exosomal non-coding RNAs in osteoarthritis. Cell Biochem Funct. 2022;40(1):49-59. [39] JAQUENOD DE GIUSTI C, SANTALLA M, DAS S. Exosomal non-coding RNAs (Exo-ncRNAs) in cardiovascular health. J Mol Cell Cardiol. 2019;137:143-151. [40] LIU Q. The emerging roles of exosomal long non-coding RNAs in bladder cancer. J Cell Mol Med. 2022;26(4):966-976. [41] XIA H, HUANG Z, LIU S, et al. Exosomal Non-Coding RNAs: Regulatory and Therapeutic Target of Hepatocellular Carcinoma. Front Oncol. 2021;11:653846. [42] VALADI H, EKSTRÖM K, BOSSIOS A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654-659. [43] ZHANG M, CHEN D, ZHANG F, et al. Serum exosomal hsa-miR-135b-5p serves as a potential diagnostic biomarker in steroid-induced osteonecrosis of femoral head. Am J Transl Res. 2020;12(5):2136-2154. [44] KUANG MJ, HUANG Y, ZHAO XG, et al. Exosomes derived from Wharton’s jelly of human umbilical cord mesenchymal stem cells reduce osteocyte apoptosis in glucocorticoid-induced osteonecrosis of the femoral head in rats via the miR-21-PTEN-AKT signalling pathway. Int J Biol Sci. 2019;15(9):1861-1871. [45] DUAN DY, TANG J, TIAN HT, et al. Adipocyte-secreted microvesicle-derived miR-148a regulates adipogenic and osteogenic differentiation by targeting Wnt5a/Ror2 pathway. Life Sci. 2021;278:119548. [46] HUANG S, LI Y, WU P, et al. microRNA-148a-3p in extracellular vesicles derived from bone marrow mesenchymal stem cells suppresses SMURF1 to prevent osteonecrosis of femoral head. J Cell Mol Med. 2020;24(19):11512-11523. [47] ZHANG X, YOU JM, DONG XJ, et al. Administration of mircoRNA-135b-reinforced exosomes derived from MSCs ameliorates glucocorticoid-induced osteonecrosis of femoral head (ONFH) in rats. J Cell Mol Med. 2020;24(23): 13973-13983. [48] FATICA A, BOZZONI I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15(1):7-21. [49] ZHENG YL, SONG G, GUO JB, et al. Interactions Among lncRNA/circRNA, miRNA, and mRNA in Musculoskeletal Degenerative Diseases. Front Cell Dev Biol. 2021;9:753931. [50] XIANG S, LI Z, WENG X. The role of lncRNA RP11-154D6 in steroid-induced osteonecrosis of the femoral head through BMSC regulation. J Cell Biochem. 2019;120(10):18435-18445. [51] LI T, XIAO K, XU Y, et al. Identification of long non‑coding RNAs expressed during the osteogenic differentiation of human bone marrow‑derived mesenchymal stem cells obtained from patients with ONFH. Int J Mol Med. 2020;46(5):1721-1732. [52] ZHANG C, YUAN S, CHEN Y, et al. Neohesperidin promotes the osteogenic differentiation of human bone marrow stromal cells by inhibiting the histone modifications of lncRNA SNHG1. Cell Cycle. 2021;20(19):1953-1966. [53] FANG B, LI Y, CHEN C, et al. Huo Xue Tong Luo capsule ameliorates osteonecrosis of femoral head through inhibiting lncRNA-Miat. J Ethnopharmacol. 2019;238:111862. [54] JIN Y, ZHU HX, WEI BF. Reduced serum and local LncRNA MALAT1 expressions are linked with disease severity in patients with non-traumatic osteonecrosis of the femoral head. Technol Health Care. 2021;29(3):479-488. [55] FU D, YANG S, LU J, et al. LncRNA NORAD promotes bone marrow stem cell differentiation and proliferation by targeting miR-26a-5p in steroid-induced osteonecrosis of the femoral head. Stem Cell Res Ther. 2021;12(1):18. [56] XU Y, JIANG Y, WANG Y, et al. LINC00473 regulated apoptosis, proliferation and migration but could not reverse cell cycle arrest of human bone marrow mesenchymal stem cells induced by a high-dosage of dexamethasone. Stem Cell Res. 2020;48:101954. [57] XU Y, JIANG Y, WANG Y, et al. LINC00473 rescues human bone marrow mesenchymal stem cells from apoptosis induced by dexamethasone through the PEBP1‑mediated Akt/Bad/Bcl‑2 signaling pathway. Int J Mol Med. 2021;47(1):171-182. [58] CHEN X, LI J, LIANG D, et al. LncRNA AWPPH participates in the development of non-traumatic osteonecrosis of femoral head by upregulating Runx2. Exp Ther Med. 2020;19(1):153-159. [59] YUAN S, ZHANG C, ZHU Y, et al. Neohesperidin Ameliorates Steroid-Induced Osteonecrosis of the Femoral Head by Inhibiting the Histone Modification of lncRNA HOTAIR. Drug Des Devel Ther. 2020;14:5419-5430. [60] YU QS, GUO WS, CHENG LM, et al. Glucocorticoids Significantly Influence the Transcriptome of Bone Microvascular Endothelial Cells of Human Femoral Head. Chin Med J (Engl). 2015;128(14):1956-1963. [61] SHI S, WU X, WANG X, et al. Differentiation of Bone Marrow Mesenchymal Stem Cells to Cardiomyocyte-Like Cells Is Regulated by the Combined Low Dose Treatment of Transforming Growth Factor-β1 and 5-Azacytidine. Stem Cells Int. 2016;2016:3816256. [62] ZHANG L, LI S, LI J, et al. LncRNA ORLNC1 Promotes Bone Marrow Mesenchyml Stem Cell Pyroptosis Induced by Advanced Glycation End Production by Targeting miR-200b-3p/Foxo3 Pathway. Stem Cell Rev Rep. 2021;17(6):2262-2275. [63] PRUUNSILD P, BENGTSON CP, BADING H. Networks of Cultured iPSC-Derived Neurons Reveal the Human Synaptic Activity-Regulated Adaptive Gene Program. Cell Rep. 2017;18(1):122-135. [64] TANG YH, YUE ZS, LI GS, et al. Effect of beta-ecdysterone on glucocorticoid-induced apoptosis and autophagy in osteoblasts. Mol Med Rep. 2018;17(1): 158-164. [65] KUANG MJ, XING F, WANG D, et al. CircUSP45 inhibited osteogenesis in glucocorticoid-induced osteonecrosis of femoral head by sponging miR-127-5p through PTEN/AKT signal pathway: Experimental studies. Biochem Biophys Res Commun. 2019;509(1):255-261. [66] XIN W, YUAN S, WANG B, et al. Hsa_circ_0066523 promotes the proliferation and osteogenic differentiation of bone mesenchymal stem cells by repressing PTEN. Bone Joint Res. 2021;10(8):526-535. [67] JIANG B, ZHU SH, ZENG JY, et al. Plasma and local expressions of CircRNA CDR1as are linked with disease severity in patients with non-traumatic osteonecrosis of femoral head. J Orthop Surg Res. 2020;15(1):592. |
| [1] | Cai Zhihao, Xie Zhaoyong. Femoral neck anteversion measurement assessment: how to establish a unified method and standard [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1448-1454. |
| [2] | Dang Yi, Du Chengyan, Yao Honglin, Yuan Nenghua, Cao Jin, Xiong Shan, Zhang Dingmei, Wang Xin. Hormonal osteonecrosis and oxidative stress [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1469-1476. |
| [3] | Tao Xin, Xu Yi, Song Zhiwen, Liu Jinbo. Hippo signaling pathway in the regulation of spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(4): 619-625. |
| [4] | Li Shihao, Li Qi, Li Zhen, Zhang Yuanyuan, Liu Miaomiao, Ouyang Yi, Xu Weiguo. Plantar pressure and gait analysis in patients with anterior cruciate ligament injury and reconstruction [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(4): 626-631. |
| [5] | Li Hui, Zhang Kun, Hao Yangquan, Feng Lei, Yang Zhi, Xu Peng, Lu Chao. Robot-assisted core decompression and bone grafting for ARCO II osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(4): 547-551. |
| [6] | Zhou Jie, Pei Xibo, Wan Qianbing. Advances and biological application of asymmetric dressings [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 434-440. |
| [7] | Chen Jingqiao, Li Ying, Meng Maohua, Xu Xingxing, Wang Qinying, Wang Huan, Lu Jing, Shu Jiayu, Dong Qiang. Research progress in platelet-rich fibrin in stomatology [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 441-446. |
| [8] | Han Tao, Hao Jianqiang, Li Wenbo, Shi Jie, Gao Qiuming. Advantages and problems of antibiotic-loaded bone cements for bone and joint infections [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 470-477. |
| [9] | Chen Feng, Ren Guowu, Zhang Xiaoyun, Chen Yueping, Shi Rusheng. Receptor activator of nuclear factor-kappa B ligand signal transduction mechanism and osteoclast activation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(2): 293-299. |
| [10] | Li Mingxiu, Wang Xuan, Yang Jie, Li Yi. An osteoarthritis model in vitro: characteristics and new design idea [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(2): 300-306. |
| [11] | Liu Yinghong, Yi Yating. Mechanism and implication of angiogenesis in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(2): 307-313. |
| [12] | Zhang Jie, Tian Ai. Advances in the signaling pathway of M2 macrophages involved in bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(2): 314-321. |
| [13] | Zhu Miaomiao, Kong Fanming, Zhao Qian. Exercise regulates lactic acid metabolism [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(2): 322-328. |
| [14] | Luo Di, Liang Xuezhen, Yan Bozhao, Li Jiacheng, Xu Bo, Li Gang. Mechanism of Bushen Huoxue Capsule in repair of bone defects due to steroid-induced osteonecrosis of the femoral head in rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(2): 184-191. |
| [15] | Wei Tengfei, He Xiaoming, Wei Yurou, Zhan Zhiwei, He Mincong, He Wei, Wei Qiushi. Differential expression of Piezo1 in osseous tissue of steroid- and alcohol-induced osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(2): 270-275. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||