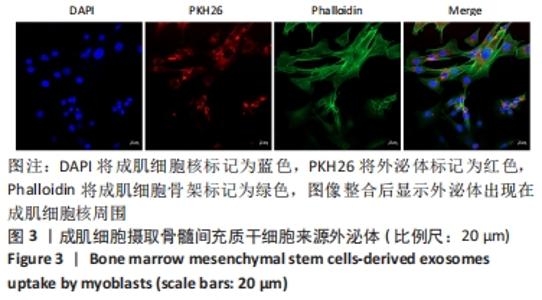
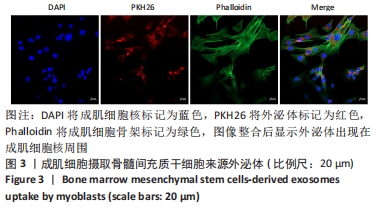
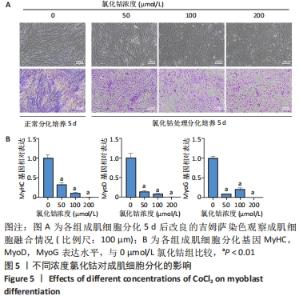
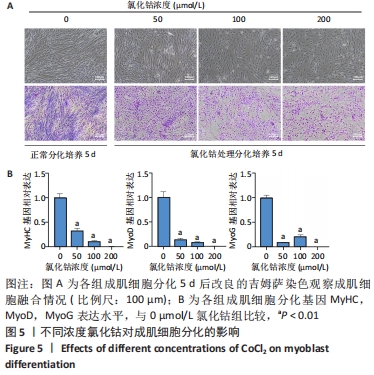
Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (6): 853-859.doi: 10.12307/2023.264
Previous Articles Next Articles
Effects of bone marrow mesenchymal stem cells-derived exosomes on hypoxia-treated myoblasts
Li Qicheng1, 2, Deng Jin2, Fu Xiaoyang1, 2, Han Na1, 2
- 1Central Laboratory, 2Department of Orthopedics and Trauma, People’s Hospital, Peking University, Beijing 100044, China
-
Received:2022-03-03Accepted:2022-05-11Online:2023-02-28Published:2022-08-11 -
Contact:Han Na, PhD, Associate researcher, Master’s supervisor, Central Laboratory, and Department of Orthopedics and Trauma, People’s Hospital, Peking University, Beijing 100044, China -
About author:Li Qicheng, Master candidate, Physician, Central Laboratory, and Department of Orthopedics and Trauma, People’s Hospital, Peking University, Beijing 100044, China -
Supported by:the National Natural Science Foundation of China, Grant No. 31671248 (to HN); Natural Science Foundation of Beijing Municipality, Grant No. 7222198 (to HN)
CLC Number:
Cite this article
Li Qicheng, Deng Jin, Fu Xiaoyang, Han Na. Effects of bone marrow mesenchymal stem cells-derived exosomes on hypoxia-treated myoblasts[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 853-859.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
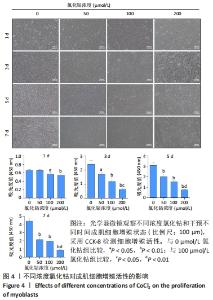
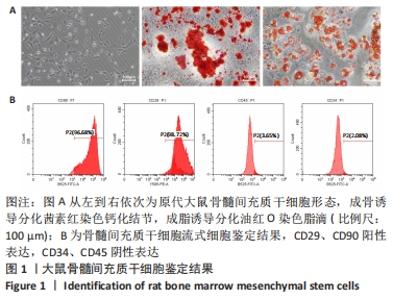
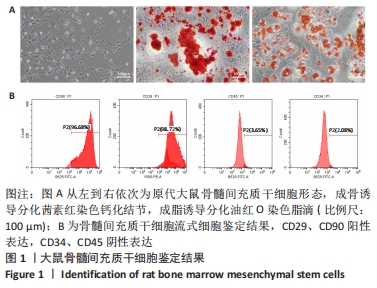
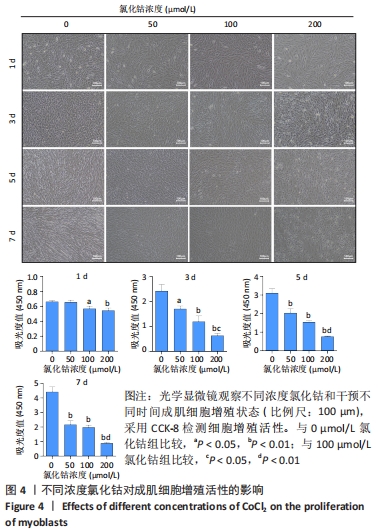
2.4 不同浓度的氯化钴对成肌细胞增殖活性的影响 分别用0,50,100,200 μmol/L氯化钴处理成肌细胞,在1,3,5,7 d后进行观察。如图4所示,与0 μmol/L 氯化钴组相比,100 μmol/L氯化钴和200 μmol/L 氯化钴处理成肌细胞1 d后细胞活性降低(P < 0.05),50 μmol/L 氯化钴组细胞活性未发生改变(P > 0.05),处理 3 d后所有氯化钴组细胞活性均显著降低,并且随着时间的延长,成肌细胞活性下降更加明显(P < 0.05)。与100 μmol/L 氯化钴组相比,200 μmol/L 氯化钴处理成肌细胞1 d后细胞活性无显著变化(P > 0.05),处理成肌细胞3 d后细胞活性显著下降(P < 0.05)。"
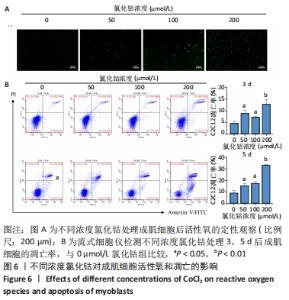
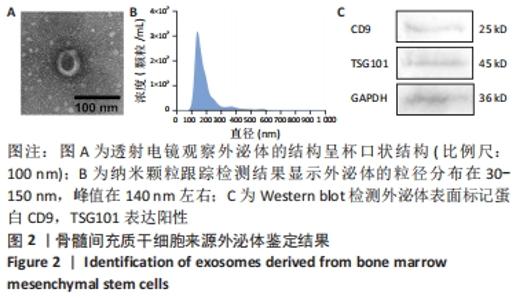
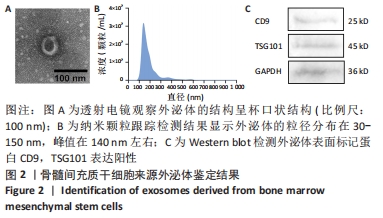
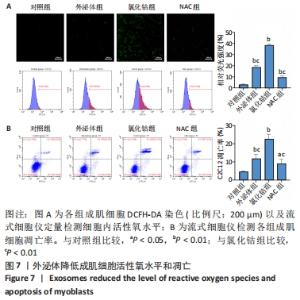
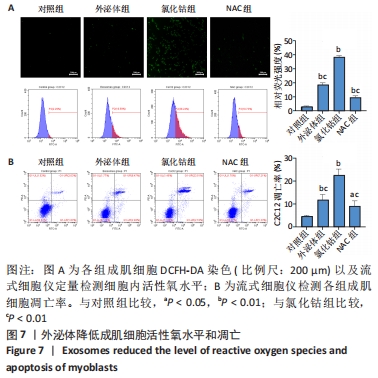
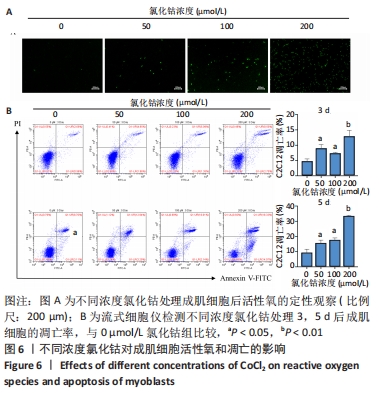
2.6 不同浓度氯化钴对成肌细胞凋亡和活性氧的影响 流式细胞检测结果显示,干预3 d后0,50,100,200 μmol/L氯化钴组凋亡率分别为(4.40±1.13)%,(8.67±1.53)%,(7.03±0.63)%,(12.67±2.17)%;干预5 d后0,50,100,200 μmol/L氯化钴组凋亡率分别为(8.62±3.03)%,(15.09± 2.52)%,(17.16±1.88)%,(32.92±1.075)%。与0 μmol/L 氯化钴组相比,50,100,200 μmol/L 氯化钴组在干预3,5 d后均能显著增加成肌细胞的凋亡率(P < 0.05),见图6。0,50,100,200 μmol/L 氯化钴处理成肌细胞3 d后进行了活性氧定性观察,发现200 μmol/L氯化钴组活性氧检测的荧光相较于其他组产生了相对的变化,为后续实验垫定基础,见图6。综合考虑,后续实验选择200 μmol/L氯化钴处理成肌细胞3 d。"
| [1] STINNER DJ, EDWARDS D. Surgical Management of Musculoskeletal Trauma. Surg Clin North Am. 2017;97(5):1119-1131. [2] GREISING SM, CORONA BT, CALL JA. Musculoskeletal Regeneration, Rehabilitation, and Plasticity Following Traumatic Injury. Int J Sports Med. 2020;41(8):495-504. [3] ARNETT TR. Acidosis, hypoxia and bone. Arch Biochem Biophys. 2010; 503(1):103-109. [4] BOGDANOVSKI DA, DIFAZIO LT, BOGDANOVSKI AK, et al. Hypoxia-inducible-factor-1 in trauma and critical care. J Crit Care. 2017;42:207-212. [5] COLLINS CA, OLSEN I, ZAMMIT PS, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122(2):289-301. [6] SCHMIDT M, SCHÜLER SC, HÜTTNER SS, et al. Adult stem cells at work: regenerating skeletal muscle. Cell Mol Life Sci. 2019;76(13):2559-2570. [7] MAQSOOD M, KANG M, WU X, et al. Adult mesenchymal stem cells and their exosomes: Sources, characteristics, and application in regenerative medicine. Life Sci. 2020;256:118002. [8] HU L, YIN C, ZHAO F, et al. Mesenchymal Stem Cells: Cell Fate Decision to Osteoblast or Adipocyte and Application in Osteoporosis Treatment. Int J Mol Sci. 2018;19(2):360. [9] NAJI A, EITOKU M, FAVIER B, et al. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci. 2019;76(17):3323-3348. [10] VOLAREVIC V, MARKOVIC BS, GAZDIC M, et al. Ethical and Safety Issues of Stem Cell-Based Therapy. Int J Med Sci. 2018;15(1):36-45. [11] TAN Y, NIE W, CHEN C, et al. Mesenchymal stem cells alleviate hypoxia-induced oxidative stress and enhance the pro-survival pathways in porcine islets. Exp Biol Med (Maywood). 2019;244(9):781-788. [12] RONG X, LIU J, YAO X, et al. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res Ther. 2019;10(1):98. [13] LIU W, LI L, RONG Y, et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020;103:196-212. [14] ZHU LP, TIAN T, WANG JY, et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 2018;8(22):6163-6177. [15] MUÑOZ-SÁNCHEZ J, CHÁNEZ-CÁRDENAS ME. The use of cobalt chloride as a chemical hypoxia model. J Appl Toxicol. 2019;39(4):556-570. [16] LUO Z, GAO Q, ZHANG H, et al. Microbe-derived antioxidants attenuate cobalt chloride-induced mitochondrial function, autophagy and BNIP3-dependent mitophagy pathways in BRL3A cells. Ecotoxicol Environ Saf. 2022;232:113219. [17] THÉRY C, AMIGORENA S, RAPOSO G, et al. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3:Unit 3.22. [18] BEST TM, HUNTER KD. Muscle injury and repair. Phys Med Rehabil Clin N Am. 2000;11(2):251-266. [19] CHU DT, PHUONG TNT, TIEN NLB, et al. An Update on the Progress of Isolation, Culture, Storage, and Clinical Application of Human Bone Marrow Mesenchymal Stem/Stromal Cells. Int J Mol Sci. 2020; 21(3):708. [20] GÓMEZ-ARNAIZ S, TATE RJ, GRANT MH. Cytotoxicity of cobalt chloride in brain cell lines - a comparison between astrocytoma and neuroblastoma cells. Toxicol In Vitro. 2020;68:104958. [21] XIAO J, ZHANG Y, ZHANG W, et al. Protective Effects of Adiponectin against Cobalt Chloride-Induced Apoptosis of Smooth Muscle Cells via cAMP/PKA Pathway. Biomed Res Int. 2020;2020:7169348. [22] CHEN Y, ZHAO Q, YANG X, et al. Effects of cobalt chloride on the stem cell marker expression and osteogenic differentiation of stem cells from human exfoliated deciduous teeth. Cell Stress Chaperones. 2019; 24(3):527-538. [23] VENGELLUR A, WOODS BG, RYAN HE, et al. Gene expression profiling of the hypoxia signaling pathway in hypoxia-inducible factor 1alpha null mouse embryonic fibroblasts. Gene Expr. 2003;11(3-4):181-197. [24] WAGATSUMA A, ARAKAWA M, MATSUMOTO H, et al. Cobalt chloride, a chemical hypoxia-mimicking agent, suppresses myoblast differentiation by downregulating myogenin expression. Mol Cell Biochem. 2020; 470(1-2):199-214. [25] JAKUBCZYK K, DEC K, KAŁDUŃSKA J, et al. Reactive oxygen species - sources, functions, oxidative damage. Pol Merkur Lekarski. 2020;48(284): 124-127. [26] LI L, TAN J, MIAO Y, et al. ROS and Autophagy: Interactions and Molecular Regulatory Mechanisms. Cell Mol Neurobiol. 2015;35(5): 615-621. [27] GUAN D, SU Y, LI Y, et al. Tetramethylpyrazine inhibits CoCl2 -induced neurotoxicity through enhancement of Nrf2/GCLc/GSH and suppression of HIF1α/NOX2/ROS pathways. J Neurochem. 2015;134(3):551-565. [28] JIANG N, ZHAO H, HAN Y, et al. HIF-1α ameliorates tubular injury in diabetic nephropathy via HO-1-mediated control of mitochondrial dynamics. Cell Prolif. 2020;53(11):e12909. [29] PANG Z, WANG T, LI Y, et al. Liraglutide ameliorates COCl2-induced oxidative stress and apoptosis in H9C2 cells via regulating cell autophagy. Exp Ther Med. 2020;19(6):3716-3722. [30] KIM RJ, AN SH, GWARK JY, et al. Antioxidant effects on hypoxia-induced oxidative stress and apoptosis in rat rotator cuff fibroblasts. Eur Cell Mater. 2021;41:680-693. [31] CHOWDHURY R, HARDY A, SCHOFIELD CJ. The human oxygen sensing machinery and its manipulation. Chem Soc Rev. 2008;37(7):1308-1319. [32] TRAMS EG, LAUTER CJ, SALEM N JR, et al. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645(1):63-70. [33] VALADI H, EKSTRÖM K, BOSSIOS A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654-659. [34] SOARES MARTINS T, TRINDADE D, VAZ M, et al. Diagnostic and therapeutic potential of exosomes in Alzheimer’s disease. J Neurochem. 2021;156(2):162-181. [35] JIA G, HAN Y, AN Y, et al. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials. 2018;178:302-316. [36] WEN Z, MAI Z, ZHU X, et al. Mesenchymal stem cell-derived exosomes ameliorate cardiomyocyte apoptosis in hypoxic conditions through microRNA144 by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020;11(1):36. [37] MAO Q, LIANG XL, ZHANG CL, et al. LncRNA KLF3-AS1 in human mesenchymal stem cell-derived exosomes ameliorates pyroptosis of cardiomyocytes and myocardial infarction through miR-138-5p/Sirt1 axis. Stem Cell Res Ther. 2019;10(1):393. |
| [1] | Fang Xingyan, Tian Zhenli, Zhao Zheyi, Wen Ping, Xie Tingting. Effects of sodium arsenite on human umbilical vein endothelial cell injury and sphingosine kinases 1/sphingosine 1-phosphate signaling axis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(在线): 1-7. |
| [2] | Pan Zhongjie, Qin Zhihong, Zheng Tiejun, Ding Xiaofei, Liao Shijie. Targeting of non-coding RNAs in the pathogenesis of the osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1441-1447. |
| [3] | Dang Yi, Du Chengyan, Yao Honglin, Yuan Nenghua, Cao Jin, Xiong Shan, Zhang Dingmei, Wang Xin. Hormonal osteonecrosis and oxidative stress [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1469-1476. |
| [4] | Nie Chenchen, Su Kaiqi, Gao Jing, Fan Yongfu, Ruan Xiaodi, Yuan Jie, Duan Zhaoyuan, Feng Xiaodong. The regulatory role of circular RNAs in cerebral ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1286-1291. |
| [5] | Xu Cong, Zhao He, Sun Yan. Regeneration of facial nerve injury repaired by biomaterial nerve conduits [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1089-1095. |
| [6] | Xue Ting, Zhang Xinri, Kong Xiaomei. Mesenchymal stem cell therapy for pneumoconiosis using nanomaterials combined with multi-modal molecular imaging [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1133-1140. |
| [7] | Yuan Wei, Liu Jingdong, Xu Guanghui, Kang Jian, Li Fuping, Wang Yingjie, Zhi Zhongzheng, Li Guanwu. Osteogenic differentiation of human perivascular stem cells and its regulation based on Wnt/beta-catenin signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 866-871. |
| [8] | Hao Liufang, Duan Hongmei, Wang Zijue, Hao Fei, Hao Peng, Zhao Wen, Gao Yudan, Yang Zhaoyang, Li Xiaoguang. Spatiotemporal dynamic changes of ependymal cells after spinal cord injury in transgenic mice [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 883-889. |
| [9] | Li Xiaoyin, Yang Xiaoqing, Chen Shulian, Li Zhengchao, Wang Ziqi, Song Zhen, Zhu Daren, Chen Xuyi. Collagen/silk fibroin scaffold combined with neural stem cells in the treatment of traumatic spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 890-896. |
| [10] | Ke Weiqiang, Chen Xianghui, Chen Xiaoling, Meng Jie, Ma Yanlin. Rituximab combined with autologous peripheral blood stem cell transplantation in the treatment of diffuse large B-cell lymphoma and the expression of related factors [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 915-920. |
| [11] | Huang Guijiang, Ji Yuwei, Zhao Xin, Yang Yi, Zhao Yulan, Wang Peijin, Tang Wei, Jiao Jianlin. Effect and mechanism of different administration routes of placenta-derived mesenchymal stem cells in the treatment of tree shrews with osteoporotic fracture [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 909-914. |
| [12] | Bai Siqi, Xiao Zhen, Liu Jing. Application potential of adipose-derived stem cells in female pelvic floor dysfunction diseases [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 921-927. |
| [13] | Zhang Qijian, Xu Ximing. Acquisition and application of ectodermal mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 928-934. |
| [14] | Yuan Bo, Xie Lide, Fu Xiumei. Schwann cell-derived exosomes promote the repair and regeneration of injured peripheral nerves [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 935-940. |
| [15] | Yin Shuoxin, Zhang Tao, Wang Shuping, Lu Xin, Huang Xuping, Yin Mengying, Yang Yuwei, Yuan Bingmao, Mao Zhihua, Chen Yuanneng. Bibliometric and visualized analysis of researches on gastric cancer stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 941-947. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||