Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (6): 941-947.doi: 10.12307/2023.229
Previous Articles Next Articles
Bibliometric and visualized analysis of researches on gastric cancer stem cells
Yin Shuoxin1, Zhang Tao2, Wang Shuping1, Lu Xin1, Huang Xuping2, Yin Mengying1, Yang Yuwei1, Yuan Bingmao1, Mao Zhihua1, Chen Yuanneng2
- 1Guangxi University of Chinese Medicine, Nanning 530001, Guangxi Zhuang Autonomous Region, China; 2Department of Gastroenterology, Ruikang Hospital Affiliated to Guangxi University of Chinese Medicine, Nanning 530011, Guangxi Zhuang Autonomous Region, China
-
Received:2022-02-21Accepted:2022-03-17Online:2023-02-28Published:2022-08-12 -
Contact:Chen Yuanneng, MD, Professor, Department of Gastroenterology, Ruikang Hospital Affiliated to Guangxi University of Chinese Medicine, Nanning 530011, Guangxi Zhuang Autonomous Region, China -
About author:Yin Shuoxin, Master candidate, Guangxi University of Chinese Medicine, Nanning 530001, Guangxi Zhuang Autonomous Region, China -
Supported by:Local Project of National Natural Science Foundation of China, No. 81360531 (to CYN); General Project of Guangxi Natural Science Foundation, No. 2020GXNSFAA238030 (to CYN); Guangxi Key First-Class Discipline Construction Project, No. 2019XK166 (to CYN); Graduate Education Innovation Plan Project of Guangxi University of Chinese Medicine, No. YCSZ2022018 (to YSX)
CLC Number:
Cite this article
Yin Shuoxin, Zhang Tao, Wang Shuping, Lu Xin, Huang Xuping, Yin Mengying, Yang Yuwei, Yuan Bingmao, Mao Zhihua, Chen Yuanneng. Bibliometric and visualized analysis of researches on gastric cancer stem cells[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 941-947.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
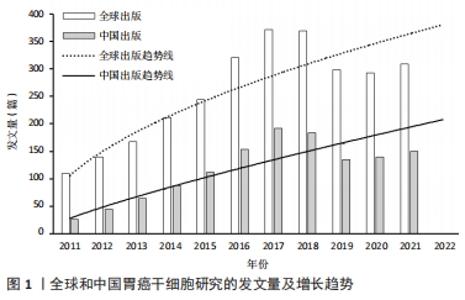
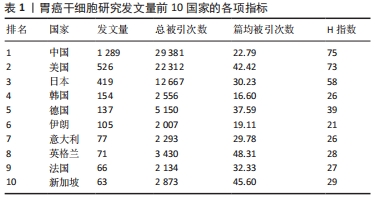
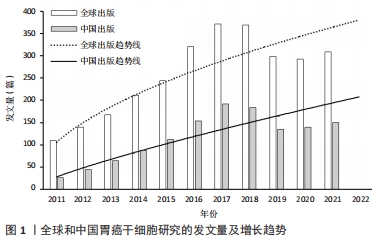
2.1 发文量的变化趋势 为了解胃癌干细胞研究领域的发展速度,对发文量随年份的变化进行可视化分析。自2011年以来胃癌干细胞方面的文献发表量逐年递增,2017年全球发文量达到最高(372篇),当年发文量占2011-2021年总发文量的13.13%,表明胃癌干细胞研究已经成为当下全球关注的焦点。全球发文量的飞速增长很大程度上得益于胃癌干细胞研究领域的快速发展。2011-2014年间,国内学者的发文量仅有222篇,占全球总发文量的7.83%。然而随着2015年前后国内进入研究的快速增长期,截至2021年国内发文量已经达到了1 289篇,占全球总发文量的45.51%,位居世界第一,说明国内学者对胃癌干细胞研究的重视程度在不断增加。利用Excel绘制2011-2021年国内外在胃癌干细胞领域发文量的变化趋势图。通过图1可以看出,自2019年开始,全球及中国胃癌干细胞相关研究成果出现了缓慢下降的趋势,但2021年稍有回升。这种现象可能与新冠疫情有关,相信在不久的将来人类终将战胜疫情,恢复科研生产力。此外通过趋势线预计2022年全球和中国发文量将会较去年有大幅提升。"

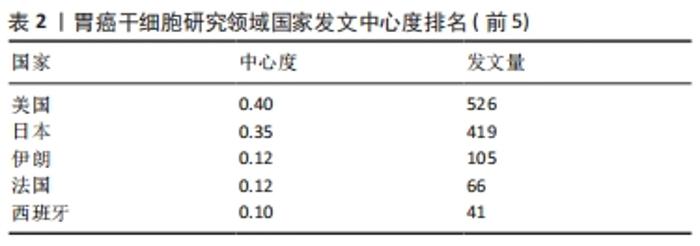
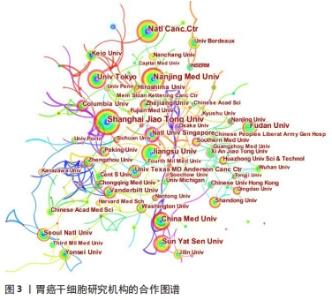
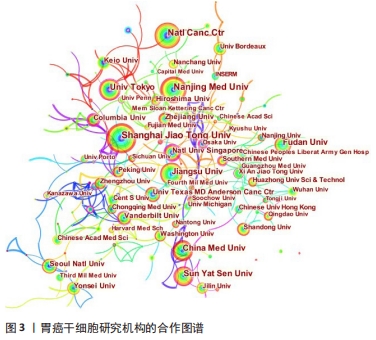
运用Citespace软件对发文国家进行共现网络分析,见图2。图内国家以不同的节点表示,直径与该国发文量呈正比;连线表示不同国家间存在合作关系;最外圈的紫色环表示该节点为中心性节点,即该国与其他国家的合作中占据重要地位,如美国和法国。美国居于胃癌干细胞研究的核心地位,与大部分国家有着学术研究合作。中国主要与美国、西班牙和加拿大的国际合作较为密切。将图2中的信息导出,形成国家发文中心度排名表,见表2。中介中心度是指网络中经过某点并连接这两点的最短路径占这两点之间的最短路径线总数之比,是用来刻画节点重要性的指标,数值越大,则说明该节点在网络中处于关键位置,影响力越大[6-7]。在文中,中介中心度则是用来反映某个国家在合作网络中的重要性。中心度排名前5的国家中,美国是最早开始探索并形成一定研究规模的国家;中国的发文量虽然居第1位,但中心度值却远远低于其他国家。综合中国的篇均被引频次和中介中心度可以看出,虽然近年来中国在胃癌干细胞领域的研究成果数量位居第一,但整体而言,成果的可靠性、扎实度和创新性与其他国家相比稍显薄弱,未来相关研究应该从数量向质量转变,进一步提高中国在世界上的学术地位。 "

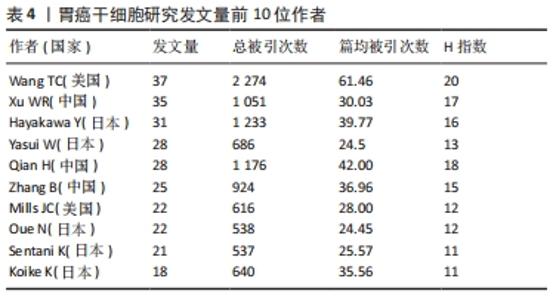
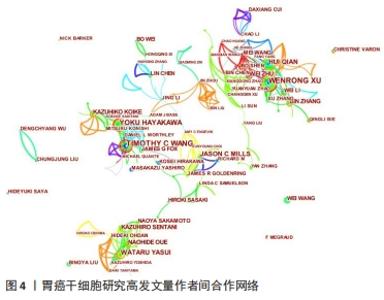
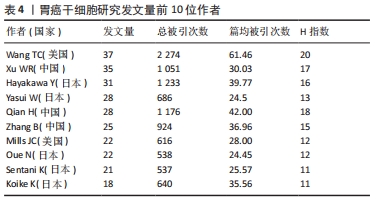
2.3 发文作者及合作关系 全球在胃癌干细胞研究领域发表文章的学者有12 958名,其中有31名学者的发文量在20篇以上。发文量前10的学者中有5名来自日本,3名来自中国,2名来自美国,见表4。美国哥伦比亚大学的Wang TC以37篇的发文量位居第一。来自中国江苏大学的许文荣(Xu WR)和来自日本东京大学的Hayakawa Y分别以35篇和31篇的发文量位列2,3位。在被引频次上,美国的Wang TC (2 274)、日本的Hayakawa Y(1 233)与中国的Qian H (1 176)位列总被引频次的前3位,展现出这几位学者在胃癌干细胞研究领域的绝对优势和影响力。从国内学者来看,江苏大学的许文荣(Xu WR)、钱晖(Qian H)和张斌(Zhang B)分别以35,28和25篇的发文量列于国内该方向发文量的前3,分别排在世界发文量的第2,5和6位,其中许文荣的总被引频次是1 051,篇均被引频次为30.03。他们与中国人民解放军总医院的卫勃(Wei B)和中国医科大学的王巍(Wang W)等均是国内胃癌干细胞研究的重要力量,在国内外均具有一定的学术影响力。"
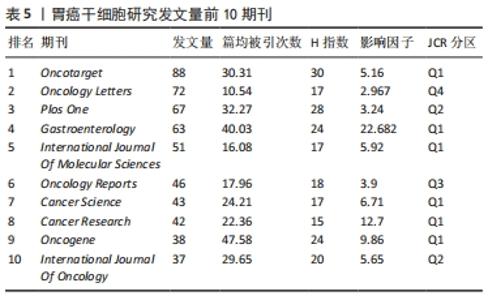
2.4 发文期刊 作为研究成果交流的媒介,期刊在促进国际合作及科研水平进步中发挥着至关重要的作用。2011-2021年间,全球出版胃癌干细胞研究的期刊有731家,其中发文量前10期刊的出版量均在30篇以上,占总发文量的19.31%,见表5。《Oncotarget》以88篇的发文量成为发表胃癌干细胞研究成果最多的期刊,占总发文量的3.10%,其次为《Oncology Letters》和《Plos One》,分别发表了72篇和67篇,占总发文量的2.54%和2.36%。此外《Oncotarget》和《PloS One》分别位居篇均被引频次和H指数的首位。在这10种期刊中,其中6种期刊为Q1分区,2种为Q2分区,Q3、Q4分区各1种,影响因子分布在2.96-22.68之间,其中《Gastroenterology》的影响因子最高。"
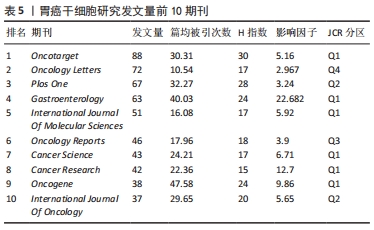
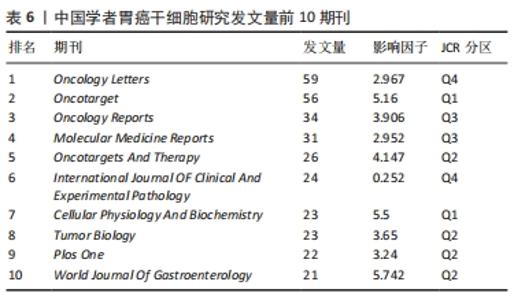
表6列举了中国学者发文量前10的期刊,《Oncology Letters》以59篇的发文量位居第一,属于Q4区,影响因子为2.96;《Oncotarget》和《Oncology Reports》的发文量分别为56篇和34篇,位列发文量的第二和第三。此外,中国学者发文量前10的期刊中,有4种期刊为Q3、Q4分区,其余期刊集中在Q1区和Q2区,影响因子分布在3.65-5.74之间,这些期刊也是中国学者发表胃癌干细胞研究成果时关注最多的国际期刊。值得注意的是,虽然《Oncotarget》曾经作为国内外学者发表胃癌干细胞研究成果最多的期刊,但其在2020年已不再被SCI收录,希望相关研究领域的科研工作者选择其他更具价值的期刊交流学术成果。 "
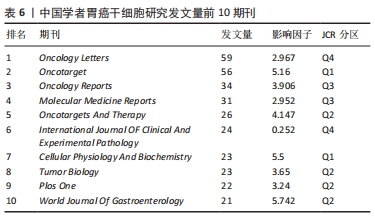
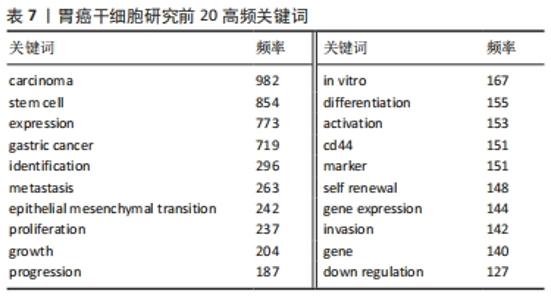
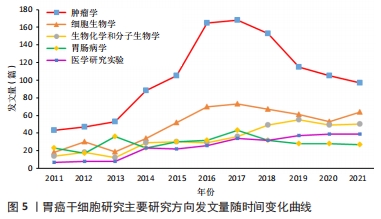
2.6 关键词分析 利用Citespace进行关键词分析,把Node types设定为Keyword,时间区间为2011-2021年,时间切片为1年,分析出现频率前20的关键词,见表7。高频关键词提示胃癌干细胞的研究与上皮间质转化、对胃癌细胞生物学功能的影响以及自我更新等密切相关。上皮间质转化是指细胞失去上皮特性,其标志是细胞间黏附丧失、肌动蛋白细胞骨架重构、获得运动性的增强、抵抗凋亡和衰老等[8]。上皮间质转化与癌症的多种恶性行为有关,如增殖、侵袭和转移等[9]。研究表明,胃癌干细胞起源于胃上皮细胞,并且在胃癌干细胞中检测出上皮间质转化标志物和转录因子,由此可以说明胃癌干细胞具有上皮间质转化的特征[10]。多项研究表明,靶向肿瘤的间充质干细胞不仅为肿瘤细胞提供微环境,还能促进肿瘤生长和转移,白细胞介素15作为主要由胃间充质干细胞分泌的多效性细胞因子,通过上调Treg和增强CD4+T细胞中PD-1的表达促进肿瘤细胞上皮间质转化[11]。近年来,肿瘤干细胞因其自我更新、致瘤性和多重分化潜能被认为与肿瘤的起源、复发和转移有关[12]。一些促进胃癌干细胞自我更新的关键分子如TAK1、YAP等均可调控胃癌干细胞的自我更新,从而干预胃癌的发生和发展[13-14]。"
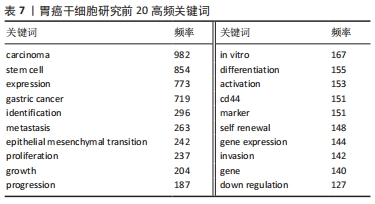

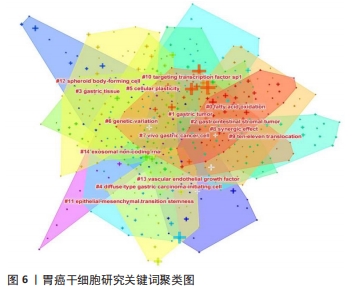
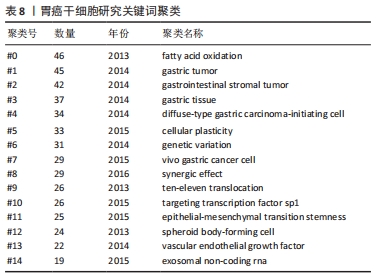
关键词的聚类分析是将彼此联系紧密的关键词进行聚类,能够反映某领域当前的研究热点。文章通过CiteSpace对关键词进行K均值聚类分析,共得出15个聚类,见表8。胃癌干细胞领域的关键词共现网络见图6。目前主要的研究方向可以归为如下几类:#1 (胃癌)、#2 (胃肠道间质瘤)、#3 (胃组织)、#4(弥漫性胃起始细胞)、#8(胃癌细胞)归为胃癌干细胞研究的主要组织和细胞;#0 (脂肪酸氧化)、#5 (细胞可塑性)、#6 (基因变异)、#9 (协同效应)、#12 (上皮间充质转化)归为胃癌干细胞的作用机制;#10(TET蛋白)、#11(靶向转录因子sp1)、#14(血管内皮生长因子)、#15 (外泌体非编码RNA)归为干预胃癌干细胞的关键分子。这15种聚类基本可以概括胃癌干细胞研究的发展进程,同时也可看出当前的研究热点。肿瘤细胞在复杂的肿瘤微环境中进行代谢重编程以支持其生长和进展,脂肪酸通过脂肪酸氧化成为重要的能量来源,已被证明是癌细胞生长和生存所需[15]。脂肪酸氧化可以通过抑制肿瘤干细胞功能从而缓解肿瘤生长,研究也表明抑制脂肪酸氧化后胃癌细胞的增殖和迁移能力明显下降[16-17]。此外,肿瘤干细胞具有很高的可塑性,这可以改变它们的表型和功能,这些变化通常是由放化疗以及衰老的肿瘤细胞引起,从而改变肿瘤微环境,在该环境中肿瘤细胞经历生长停滞和免疫细胞被吸引阶段,最终达到抑制肿瘤生长的目的,除了这些积极的作用外,治疗后衰老的肿瘤细胞也会表现出消极作用,其通过增加肿瘤干细胞的可塑性、激活非肿瘤干细胞中的干细胞通路、促进衰老逃逸及随后的干细胞通路激活等方式直接激活肿瘤干细胞,最终导致肿瘤复发和转移[18]。新生血管供应或血管生成在维持机体平衡的发展中起着至关重要的作用,血管将营养物质输送到组织和器官,并清除分解代谢产物。 然而,不受控制的血管生长可以促进许多疾病的发生和发展,包括肿瘤,血液供应能够促进癌症的进展、复发和转移[19]。在肿瘤生长过程中,血管生成是保证氧气和营养物质运输到肿瘤的必要条件,血管内皮生长因子是血管生成的主要诱导因子,其通过直接相互作用和调节内皮细胞蛋白表达或血管通透性,间接调节先天和适应性免疫反应,是抗肿瘤免疫反应的关键调节因子[20]。血管龛是维持干细胞表型的关键,如正常干细胞的自我更新、未分化状态和休眠等[21]。肿瘤干细胞的自我更新和肿瘤的启动伴随着血管生成的促进,分泌促血管生成因子,如血管内皮生长因子,然而,血管生成并不是肿瘤营养和氧气的唯一来源,这一过程被称为血管生成拟态,存在于不同类型的癌症中,并负责为肿瘤组织提供充足的血液供应[22]。此外,肿瘤干细胞还能够通过表达血管内皮生长因子来修饰肿瘤微环境,从而促进肿瘤新生血管的形成,最终促进肿瘤的生长[23]。最新证据表明,不同类型的非编码RNA,如miRNA和lncRNA,能够调节肿瘤干细胞中激活的转录因子和下游信号通路,在肿瘤干细胞的生长和复制中发挥作用[24]。miRNA是一种21-25个核苷酸长的非编码RNA,可促进mRNA裂解,并通过与mRNA的3’UTRs结合抑制mRNA的翻译[25]。特异性miRNA的异常表达与白血病、乳腺癌、胃癌、结肠癌、肝癌、肺癌等多种癌症的发生发展密切相关,有证据表明,miRNA通过促进或抑制调节肿瘤干细胞的癌变、分化和上皮间质转化的通路来维持肿瘤干细胞的干性[24]。最近描述了miR-501-5p在胃癌细胞株和胃癌患者标本中上调,作为Wnt/β-catenin信号通路的激活剂,增强了肿瘤干细胞表型[26]。此外,由于lncRNA在细胞进程中的关键作用,其已成为癌症研究的热点。最近有研究发现lncRNA作为未分化肿瘤干细胞的生物标志物,这些lncRNA可能很容易被用来检测残留的肿瘤干细胞,从而检测肿瘤复发的风险[27]。最近的一些报道已经预示了lncRNA在肿瘤干细胞生物学中的重要性。例如lncRNA FEZF1-AS1在胃癌组织和细胞系中表达上调,FEZF1-AS1沉默抑制了胃癌干细胞的增殖、活力、侵袭和迁移[28];lncRNA H19在胃癌组织和胃癌干细胞中高表达,其可能通过作用于干细胞相关蛋白Sox2促进胃癌干细胞的增殖和转移[29]。目前越来越多的证据表明非编码RNA在癌症进展和肿瘤干细胞生物学过程中发挥重要作用,在肿瘤的生长和转移过程中同时作为肿瘤的抑制因子或启动子,它们在组织中的选择性表达使得靶向miRNA和lncRNA成为潜在治疗策略。 "
| [1] SUNG H, FERLAY J, SIEGEL RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249. [2] JIN G, LV J, YANG M, et al. Genetic risk, incident gastric cancer, and healthy lifestyle: a meta-analysis of genome-wide association studies and prospective cohort study. Lancet Oncol. 2020;21(10):1378-1386. [3] MACHLOWSKA J, BAJ J, SITARZ M, et al. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci. 2020;21(11):4012. [4] 陈彦臻,刘沈林,邹玺.胃癌干细胞与上皮间质转化研究进展[J].中华肿瘤防治杂志,2018,25(10):750-754. [5] 陈悦,陈超美,刘则渊,等.CiteSpace知识图谱的方法论功能[J].科学学研究,2015,33(2):242-253. [6] 尹硕鑫,张涛,卢鑫,等.粪菌移植研究的文献计量学和可视化分析[J/OL].微生物学通报,https://doi.org/10.13344/j.microbiol.china.211109. [7] 肖鹏飞.全球黄曲霉毒素研究的文献计量学分析[J/OL].食品科学,http://kns.cnki.net/kcms/detail/11.2206.TS.20210911.0958.010.html. [8] ZHANG N, NG AS, CAI S, et al. Novel therapeutic strategies: targeting epithelial-mesenchymal transition in colorectal cancer. Lancet Oncol. 2021;22(8):e358-e368. [9] PAN G, LIU Y, SHANG L, et al. EMT-associated microRNAs and their roles in cancer stemness and drug resistance. Cancer Commun (Lond). 2021;41(3):199-217. [10] ZHANG S, SHANG Y, CHEN T, et al. Human circulating and tissue gastric cancer stem cells display distinct epithelial-mesenchymal features and behaviors. J Cancer Res Clin Oncol. 2017;143(9):1687-1699. [11] SUN L, WANG Q, CHEN B, et al. Human Gastric Cancer Mesenchymal Stem Cell-Derived IL15 Contributes to Tumor Cell Epithelial-Mesenchymal Transition via Upregulation Tregs Ratio and PD-1 Expression in CD4+T Cell. Stem Cells Dev. 2018;27(17):1203-1214. [12] SUN LF, YANG K, WANG YG, et al. The Role of HER2 in Self-Renewal, Invasion, and Tumorigenicity of Gastric Cancer Stem Cells. Front Oncol. 2020;10:1608. [13] BIE Q, LI X, LIU S, et al. YAP promotes self-renewal of gastric cancer cells by inhibiting expression of L-PTGDS and PTGDR2. Int J Clin Oncol. 2020;25(12):2055-2065. [14] WANG G, SUN Q, ZHU H, et al. The stabilization of yes-associated protein by TGFβ-activated kinase 1 regulates the self-renewal and oncogenesis of gastric cancer stem cells. J Cell Mol Med. 2021;25(14): 6584-6601. [15] WU L, ZHANG X, ZHENG L, et al. RIPK3 Orchestrates Fatty Acid Metabolism in Tumor-Associated Macrophages and Hepatocarcinogenesis. Cancer Immunol Res. 2020;8(5):710-721. [16] HE W, LIANG B, WANG C, et al. MSC-regulated lncRNA MACC1-AS1 promotes stemness and chemoresistance through fatty acid oxidation in gastric cancer. Oncogene. 2019;38(23):4637-4654. [17] WANG L, LI C, SONG Y, et al. Inhibition of carnitine palmitoyl transferase 1A-induced fatty acid oxidation suppresses cell progression in gastric cancer. Arch Biochem Biophys. 2020;696:108664. [18] WALCHER L, KISTENMACHER AK, SUO H, et al. Cancer Stem Cells-Origins and Biomarkers: Perspectives for Targeted Personalized Therapies. Front Immunol. 2020;11:1280. [19] APTE RS, CHEN DS, FERRARA N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell. 2019;176(6):1248-1264. [20] GEINDREAU M, GHIRINGHELLI F, BRUCHARD M. Vascular Endothelial Growth Factor, a Key Modulator of the Anti-Tumor Immune Response. Int J Mol Sci. 2021;22(9):4871. [21] COMAZZETTO S, SHEN B, MORRISON SJ. Niches that regulate stem cells and hematopoiesis in adult bone marrow. Dev Cell. 2021;56(13):1848-1860. [22] LIZÁRRAGA-VERDUGO E, AVENDAÑO-FÉLIX M, BERMÚDEZ M, et al. Cancer Stem Cells and Its Role in Angiogenesis and Vasculogenic Mimicry in Gastrointestinal Cancers. Front Oncol. 2020;10:413. [23] GARZA TREVIÑO EN, GONZÁLEZ PD, VALENCIA SALGADO CI, et al. Effects of pericytes and colon cancer stem cells in the tumor microenvironment. Cancer Cell Int. 2019;19:173. [24] YAN H, BU P. Non-coding RNAs in cancer stem cells. Cancer Lett. 2018; 421:121-126. [25] STAVAST CJ, ERKELAND SJ. The Non-Canonical Aspects of MicroRNAs: Many Roads to Gene Regulation. Cells. 2019;8(11):1465. [26] FAN D, REN B, YANG X, et al. Upregulation of miR-501-5p activates the wnt/β-catenin signaling pathway and enhances stem cell-like phenotype in gastric cancer. J Exp Clin Cancer Res. 2016;35(1):177. [27] RASMUSSEN TP. Parallels between artificial reprogramming and the biogenesis of cancer stem cells: Involvement of lncRNAs. Semin Cancer Biol. 2019;57:36-44. [28] HUI Y, YANG Y, LI D, et al. LncRNA FEZF1-AS1 Modulates Cancer Stem Cell Properties of Human Gastric Cancer Through miR-363-3p/HMGA2. Cell Transplant. 2020;29:963689720925059. [29] 夏荣钧,欧英富,邢维山,等.长链非编码RNA H19促进胃癌干细胞的增殖和转移[J].中国组织工程研究,2019,23(13):2022-2027. [30] LIM JR, MOUAWAD J, GORTON OK, et al. Cancer stem cell characteristics and their potential as therapeutic targets. Med Oncol. 2021;38(7):76. [31] BECERRIL-RICO J, ALVARADO-ORTIZ E, TOLEDO-GUZMÁN ME, et al. The cross talk between gastric cancer stem cells and the immune microenvironment: a tumor-promoting factor. Stem Cell Res Ther. 2021;12(1):498. [32] 代俊泽,金海峰.胃癌干细胞标志物的研究进展[J].中国老年学杂志,2020,40(24):5364-5371. [33] 李向辉,王贵吉.胃癌干细胞CSC-G在胃癌侵袭和转移中的作用[J].中国组织工程研究,2016,20(11):1597-1602. [34] SONBOL MB, AHN DH, BEKAII-SAAB T. Therapeutic Targeting Strategies of Cancer Stem Cells in Gastrointestinal Malignancies. Biomedicines. 2019;7(1):17. [35] RUGGIERI V, RUSSI S, ZOPPOLI P, et al. The Role of MicroRNAs in the Regulation of Gastric Cancer Stem Cells: A Meta-Analysis of the Current Status. J Clin Med. 2019;8(5):639. [36] CHEN B, CAI T, HUANG C, et al. G6PD-NF-κB-HGF Signal in Gastric Cancer-Associated Mesenchymal Stem Cells Promotes the Proliferation and Metastasis of Gastric Cancer Cells by Upregulating the Expression of HK2. Front Oncol. 2021;11:648706. [37] LI W, ZHANG X, WU F, et al. Gastric cancer-derived mesenchymal stromal cells trigger M2 macrophage polarization that promotes metastasis and EMT in gastric cancer. Cell Death Dis. 2019;10(12):918. [38] 张丽贤,张宁,袁双珍,等.胃癌干细胞在肿瘤侵袭转移及对血管形成的影响[J].中国组织工程研究,2016,20(32): 4738-4744. [39] HUANG C, YUAN W, LAI C, et al. EphA2-to-YAP pathway drives gastric cancer growth and therapy resistance. Int J Cancer. 2020;146(7):1937-1949. [40] CHOI HJ, JHE YL, KIM J, et al. FoxM1-dependent and fatty acid oxidation-mediated ROS modulation is a cell-intrinsic drug resistance mechanism in cancer stem-like cells. Redox Biol. 2020;36:101589. [41] CARLI ALE, AFSHAR-STERLE S, RAI A, et al. Cancer stem cell marker DCLK1 reprograms small extracellular vesicles toward migratory phenotype in gastric cancer cells. Proteomics. 2021;21(13-14): e2000098. [42] HASSN MESRATI M, SYAFRUDDIN SE, MOHTAR MA, et al. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules. 2021; 11(12):1850. [43] BEKAII-SAAB T, EL-RAYES B. Identifying and targeting cancer stem cells in the treatment of gastric cancer. Cancer. 2017;123(8):1303-1312. [44] SOLEIMANI A, DADJOO P, AVAN A, et al. Emerging roles of CD133 in the treatment of gastric cancer, a novel stem cell biomarker and beyond. Life Sci. 2022;293:120050. [45] HWANG GR, YUEN JG, JU J. Roles of microRNAs in Gastrointestinal Cancer Stem Cell Resistance and Therapeutic Development. Int J Mol Sci. 2021;22(4):1624. |
| [1] | Pan Zhongjie, Qin Zhihong, Zheng Tiejun, Ding Xiaofei, Liao Shijie. Targeting of non-coding RNAs in the pathogenesis of the osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1441-1447. |
| [2] | Nie Chenchen, Su Kaiqi, Gao Jing, Fan Yongfu, Ruan Xiaodi, Yuan Jie, Duan Zhaoyuan, Feng Xiaodong. The regulatory role of circular RNAs in cerebral ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1286-1291. |
| [3] | Hu Xinming, Qiao Yanhua, Wang Xiaofan, Li Linyu, Zhao Bing. Mechanism of long non-coding RNA plasmacytoma variant translocation 1 involved in pelvic organ prolapse [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 669-675. |
| [4] | Li Zhichao, Tan Guoqing, Su Hui, Xu Zhanwang, Xue Haipeng. Regulatory role of non-coding RNAs as potential therapeutic targets in spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 758-764. |
| [5] | Shao Zichen, Li Huanan, Gu Bing, Zhang Xiaoyun, Sun Weikang, Liu Yongqian, Gan Bin. MicroRNA, long non-coding RNA and circular RNA mediate the mechanism of decreasing uric acid, anti-inflammation and regulating bone metabolism in gout [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 765-771. |
| [6] | Liu Yinghong, Yi Yating. Mechanism and implication of angiogenesis in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(2): 307-313. |
| [7] | Huang Bin, Zheng Jinxu, Zhang Jun. Progress of non-coding RNAs in stem cell abnormality of idiopathic pulmonary fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(1): 130-137. |
| [8] | Zhao Jufen, Ma Rong, Cao Jia, Yu Chuanyang, Tao Xiang, Wang Jia, Wang Libin. Regulation of peroxisome proliferator-activated receptor gamma coactivator 1 beta on stemness expression in breast cancer stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(1): 59-65. |
| [9] | Li Zhiyi, He Pengcheng, Bian Tianyue, Xiao Yuxia, Gao Lu, Liu Huasheng. Bibliometric and visualized analysis of ferroptosis mechanism research [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1202-1209. |
| [10] | Zhao Jing, Liu Xiaobo, Zhang Yue, Zhang Jiaming, Zhong Dongling, Li Juan, Jin Rongjiang. Visualization analysis of neuromuscular electrical stimulation therapy based on CiteSpace: therapeutic effects, hot spots, and developmental trends [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1234-1241. |
| [11] | Fan Jilin, Zhu Tingting, Tian Xiaoling, Liu Sijia, Su Jing, Zhang Shiliang. Effect and mechanism of non-coding RNA regulating autophagy in myocardial ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(35): 5716-5723. |
| [12] | She Jian, Zhao Jing, Zhang Jiaming, Xia Haisha, Zhong Dongling, Li Yuxi, Zheng Zhong, Li Juan, Jin Rongjiang. Visualization analysis of literature on eye tracking in cognitive behaviors of autistic patients [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(35): 5724-5732. |
| [13] | Kang Teng, Lan Fengjun, Qin Hao, Liu Gang. Long non-coding RNA and osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(32): 5242-5247. |
| [14] | Wang Dandan, Yu Yabin, Liu Shiqi, Yan Yulou, Zhang Jianhuai. Role of long non-coding RNA nuclear-enriched abundant transcript 1 in the differentiation of human umbilical cord mesenchymal stem cells into hepatocytes [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(30): 4847-4851. |
| [15] | He Qize, Meng Lin, Wang Jie, Weng Chenyi. Citespace-based visual analysis of research literature related to cartilage repair using cartilage precursor cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(29): 4723-4728. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||