Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (1): 59-65.doi: 10.12307/2023.218
Previous Articles Next Articles
Regulation of peroxisome proliferator-activated receptor gamma coactivator 1 beta on stemness expression in breast cancer stem cells
Zhao Jufen1, Ma Rong2, Cao Jia2, Yu Chuanyang1, Tao Xiang1, Wang Jia2, Wang Libin2
- 1Clinical Medicine College, Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China; 2National Biochip Research Center Sub-Center in Ningxia, General Hospital of Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China
-
Received:2021-12-06Accepted:2022-01-28Online:2023-01-08Published:2022-06-06 -
Contact:Wang Libin, MD, Researcher, National Biochip Research Center Sub-Center in Ningxia, General Hospital of Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China -
About author:Zhao Jufen, Master candidate, Clinical Medicine College, Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China -
Supported by:National Natural Science Foundation of China, No. 81860470 (to WLB); Ningxia High-Level Science and Technology Innovation Leading Talents Project, No. KJT2019003 (to WLB); Foreign Science and Technology Cooperation Project of Key Ningxia Research and Development Program, No. 2019BFH02012 (to WLB)
CLC Number:
Cite this article
Zhao Jufen, Ma Rong, Cao Jia, Yu Chuanyang, Tao Xiang, Wang Jia, Wang Libin. Regulation of peroxisome proliferator-activated receptor gamma coactivator 1 beta on stemness expression in breast cancer stem cells[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(1): 59-65.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
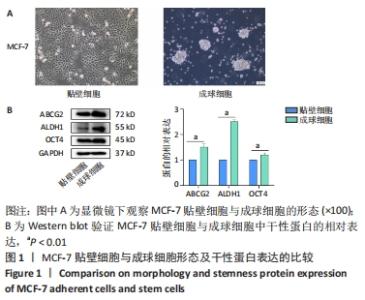
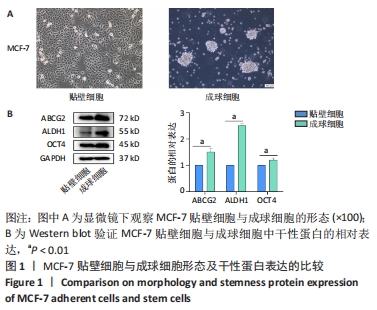
2.1 乳腺肿瘤细胞MCF-7成球能力 为了研究PGC-1β与乳腺肿瘤干细胞形成及干性蛋白表达的相关性,观察了乳腺肿瘤MCF-7贴壁细胞与成球细胞的形态变化并检测了干性相关蛋白的表达。图1A显示,显微镜下观察MCF-7细胞经过复苏培养后的贴壁细胞形态,可见细胞排列紧密,呈多边形上皮样,生长良好的MCF-7细胞经干性培养基培养后可见折光度好、中间密度高的致密乳腺癌干细胞球体。图1B显示,Western blot检测乳腺癌MCF-7细胞与乳腺癌干细胞中干性相关蛋白ABCG2、ALDH1和OCT4 的表达,结果显示,与贴壁细胞相比,成球的乳腺癌干细胞中ABCG2、ALDH1、OCT4干性蛋白的表达有明显差异性(P < 0.05)。"
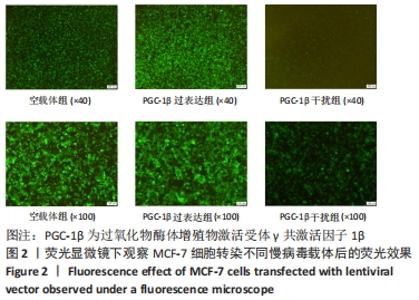
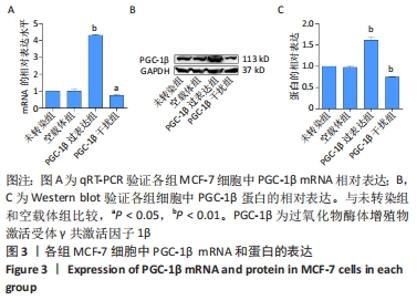
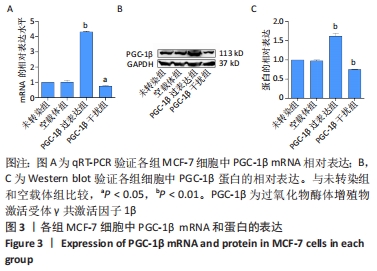
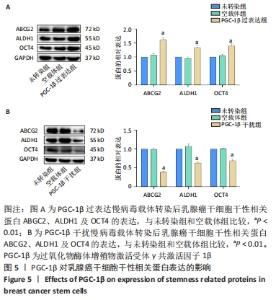
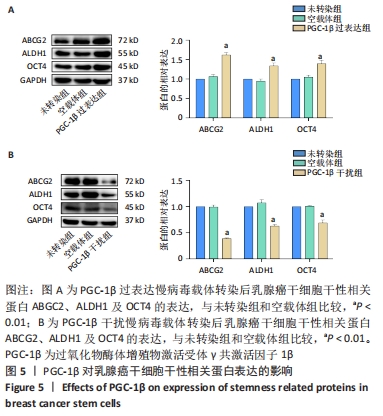
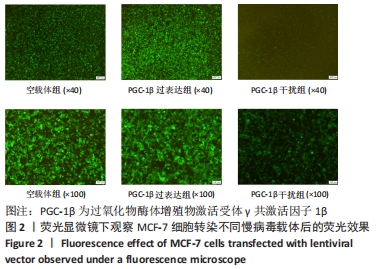
2.2 PGC-1β慢病毒载体转染MCF-7细胞鉴定结果 为了验证PGC-1β对乳腺癌干细胞干性的影响及其机制,将慢病毒载体转入乳腺癌MCF-7细胞中,荧光显微镜下观察转染PGC-1β后的MCF-7细胞,见图2,细胞荧光显示转染效率均在60%以上。经过qRT-PCR验证,过表达组PGC-1β基因的表达明显高于未转染组和空载体组,而干扰组PGC-1β基因的表达则明显低于未转染组和空载体组,见图3A;经Western blot验证,过表达组PGC-1β蛋白的表达明显高于未转染组和空载体组,而干扰组PGC-1β的表达明显低于未转染组和空载体组,见图3B,C;表明慢病毒转染成功并在MCF-7细胞中稳定表达(P < 0.05)。"
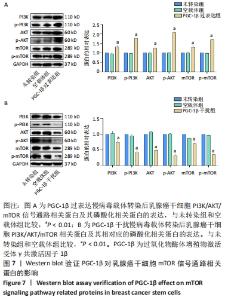
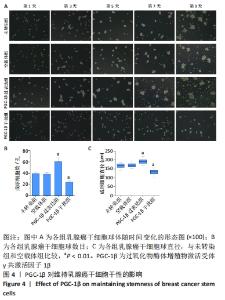
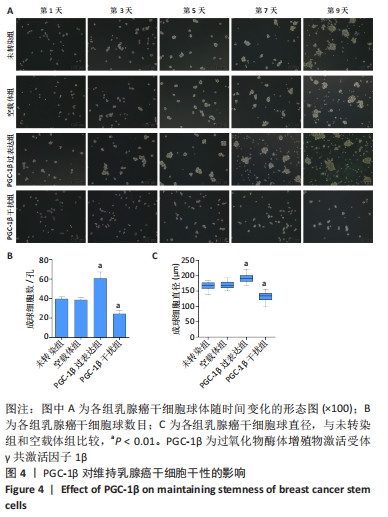
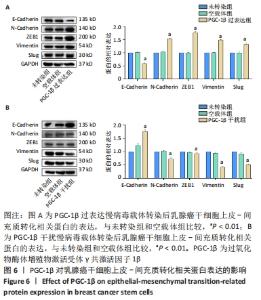
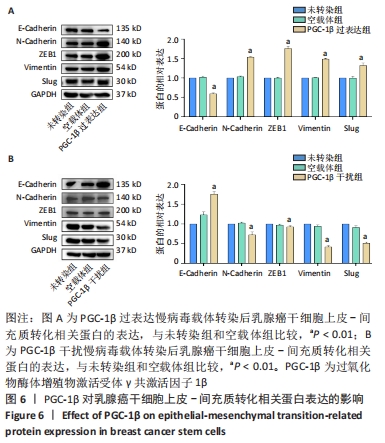
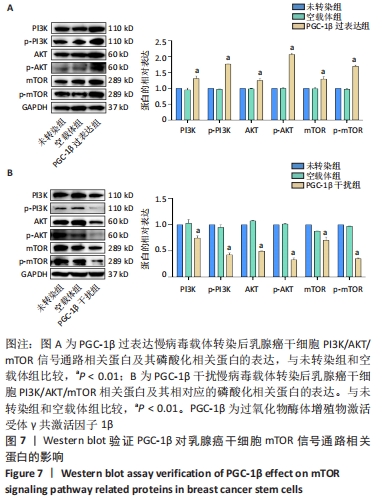
2.6 PGC-1β通过激活PI3K/AKT/mTOR通路调控乳腺癌干细胞干性表达 根据上述实验结果,PGC-1β能够通过影响上皮-间充质转化转换而影响乳腺癌干细胞的干性。进一步通过Western blot检测了PGC-1β转染后乳腺癌干细胞PI3K/AKT/mTOR 信号通路相关蛋白的表达,结果显示,与未转染组和空载体组相比,PGC-1β过表达组PI3K、AKT、mTOR及相对应的磷酸化蛋白p-PI3K、p-AKT、p-mTOR的表达显著升高,见图7A。PGC-1β干扰组上述蛋白表达降低,见图7B。结果提示PGC-1β通过激活PI3K/AKT/mTOR 信号通路促进乳腺癌干细胞的干性表达。"
| [1] SUNG H, FERLAY J, SIEGEL RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249. [2] 国家肿瘤质控中心乳腺癌专家委员会, 中国抗癌协会乳腺癌专业委员会, 中国抗癌协会肿瘤药物临床研究专业委员会. 中国晚期乳腺癌规范诊疗指南(2020版)[J].中华肿瘤杂志,2020,42(10):781-797. [3] HE L, WICK N, GERMANS SK, et al. The Role of Breast Cancer Stem Cells in Chemoresistance and Metastasis in Triple-Negative Breast Cancer. Cancers (Basel). 2021;13(24):6209. [4] WU HJ, CHU PY. Epigenetic Regulation of Breast Cancer Stem Cells Contributing to Carcinogenesis and Therapeutic Implications. Int J Mol Sci. 2021;22(15):8113. [5] MARIMA R, FRANCIES FZ, HULL R, et al. MicroRNA and Alternative mRNA Splicing Events in Cancer Drug Response/Resistance: Potent Therapeutic Targets. Biomedicines. 2021;9(12):1818. [6] ZENG X, LIU C, YAO J, et al. Breast cancer stem cells, heterogeneity, targeting therapies and therapeutic implications. Pharmacol Res. 2021; 163:105320. [7] PICCININ E, PERES C, BELLAFANTE E, et al. Hepatic peroxisome proliferator-activated receptor γ coactivator 1β drives mitochondrial and anabolic signatures that contribute to hepatocellular carcinoma progression in mice. Hepatology. 2018;67(3):884-898. [8] KELLY DP, SCARPULLA RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18(4):357-368. [9] DUCHEIX S, VEGLIANTE MC, VILLANI G, et al. Is hepatic lipogenesis fundamental for NAFLD/NASH? A focus on the nuclear receptor coactivator PGC-1β. Cell Mol Life Sci. 2016;73(20):3809-3822. [10] BELLAFANTE E, MURZILLI S, SALVATORE L, et al. Hepatic-specific activation of peroxisome proliferator-activated receptor γ coactivator-1β protects against steatohepatitis. Hepatology. 2013;57(4):1343-1356. [11] LI G, TAN X, ZHANG B, et al. Hengshun Aromatic Vinegar Improves Glycolipid Metabolism in Type 2 Diabetes Mellitus via Regulating PGC-1α/PGC-1β Pathway. Front Pharmacol. 2021;12:641829. [12] EDLING CE, FAZMIN IT, CHADDA KR, et al. Ageing in Pgc-1β-/- mice modelling mitochondrial dysfunction induces differential expression of a range of genes regulating ventricular electrophysiology. Biosci Rep. 2019;39(4):BSR20190127. [13] ZHAI M, LIU Z, ZHANG B, et al. Melatonin protects against the pathological cardiac hypertrophy induced by transverse aortic constriction through activating PGC-1β: In vivo and in vitro studies. J Pineal Res. 2017;63(3). doi: 10.1111/jpi.12433. [14] WANG L, LIU Q, LI F, et al. Apoptosis induced by PGC-1β in breast cancer cells is mediated by the mTOR pathway. Oncol Rep. 2013;30(4): 1631-1638. [15] CAO J, WANG X, WANG D, et al. PGC-1β cooperating with FOXA2 inhibits proliferation and migration of breast cancer cells. Cancer Cell Int. 2019;19:93. [16] RUHEN O, QU X, JAMALUDDIN MFB, et al. Dynamic Landscape of Extracellular Vesicle-Associated Proteins Is Related to Treatment Response of Patients with Metastatic Breast Cancer. Membranes (Basel). 2021;11(11):880. [17] MALEBARI AM, WANG S, GREENE TF, et al. Synthesis and Antiproliferative Evaluation of 3-Chloroazetidin-2-ones with Antimitotic Activity: Heterocyclic Bridged Analogues of Combretastatin A-4. Pharmaceuticals (Basel). 2021;14(11):1119. [18] NAZ F, SHI M, SAJID S, et al. Cancer stem cells: a major culprit of intra-tumor heterogeneity. Am J Cancer Res. 2021;11(12):5782-5811. [19] ZHANG T, ZHOU H, WANG K, et al. Role, molecular mechanism and the potential target of breast cancer stem cells in breast cancer development. Biomed Pharmacother. 2022;147:112616. [20] DENG J, BAI X, FENG X, et al. Inhibition of PI3K/Akt/mTOR signaling pathway alleviates ovarian cancer chemoresistance through reversing epithelial-mesenchymal transition and decreasing cancer stem cell marker expression. BMC Cancer. 2019;19(1):618. [21] BAHENA-OCAMPO I, ESPINOSA M, CEBALLOS-CANCINO G, et al. miR-10b expression in breast cancer stem cells supports self-renewal through negative PTEN regulation and sustained AKT activation. EMBO Rep. 2016;17(5):648-658. [22] VANDER HEIDEN MG, CANTLEY LC, THOMPSON CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029-1033. [23] ANDRZEJEWSKI S, KLIMCAKOVA E, JOHNSON RM, et al. PGC-1α Promotes Breast Cancer Metastasis and Confers Bioenergetic Flexibility against Metabolic Drugs. Cell Metab. 2017;26(5):778-787.e5. [24] ANDERSON AS, ROBERTS PC, FRISARD MI, et al. Ovarian tumor-initiating cells display a flexible metabolism. Exp Cell Res. 2014;328(1):44-57. [25] GLEYZER N, SCARPULLA RC. Activation of a PGC-1-related coactivator (PRC)-dependent inflammatory stress program linked to apoptosis and premature senescence. J Biol Chem. 2013;288(12):8004-8015. [26] PUIGSERVER P, WU Z, PARK CW, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6): 829-839. [27] SINGH F, ZOLL J, DUTHALER U, et al. PGC-1β modulates statin-associated myotoxicity in mice. Arch Toxicol. 2019;93(2):487-504. [28] CHAMBERS JM, WINGERT RA. PGC-1α in Disease: Recent Renal Insights into a Versatile Metabolic Regulator. Cells. 2020;9(10):2234. [29] VICTORINO VJ, BARROSO WA, ASSUNÇÃO AK, et al. PGC-1β regulates HER2-overexpressing breast cancer cells proliferation by metabolic and redox pathways. Tumour Biol. 2016;37(5):6035-6044. [30] LI Y, KASIM V, YAN X, et al. Yin Yang 1 facilitates hepatocellular carcinoma cell lipid metabolism and tumor progression by inhibiting PGC-1β-induced fatty acid oxidation. Theranostics. 2019;9(25):7599-7615. [31] WANG H, YAN X, JI LY, et al. miR-139 Functions as An Antioncomir to Repress Glioma Progression Through Targeting IGF-1 R, AMY-1, and PGC-1β. Technol Cancer Res Treat. 2017;16(4):497-511. [32] LAMBERT AW, WEINBERG RA. Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat Rev Cancer. 2021;21(5):325-338. [33] DASGUPTA A, SAWANT MA, KAVISHWAR G, et al. AECHL-1 targets breast cancer progression via inhibition of metastasis, prevention of EMT and suppression of Cancer Stem Cell characteristics. Sci Rep. 2016;6:38045. [34] GONG C, ZOU J, ZHANG M, et al. Upregulation of MGP by HOXC8 promotes the proliferation, migration, and EMT processes of triple-negative breast cancer. Mol Carcinog. 2019;58(10):1863-1875. [35] ACUÑA RA, VARAS-GODOY M, HERRERA-SEPULVEDA D, et al. Connexin46 Expression Enhances Cancer Stem Cell and Epithelial-to-Mesenchymal Transition Characteristics of Human Breast Cancer MCF-7 Cells. Int J Mol Sci. 2021;22(22):12604. [36] LI H, PREVER L, HIRSCH E, et al. Targeting PI3K/AKT/mTOR Signaling Pathway in Breast Cancer. Cancers (Basel). 2021;13(14):3517. [37] WANG L, YANG M, JIN H. PI3K/AKT phosphorylation activates ERRα by upregulating PGC‑1α and PGC‑1β in gallbladder cancer. Mol Med Rep. 2021;24(2):613. [38] MCKENNA M, MCGARRIGLE S, PIDGEON GP. The next generation of PI3K-Akt-mTOR pathway inhibitors in breast cancer cohorts. Biochim Biophys Acta Rev Cancer. 2018;1870(2):185-197. [39] LEE YR, CHEN M, PANDOLFI PP. The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat Rev Mol Cell Biol. 2018;19(9):547-562. [40] JUNG MJ, RHO JK, KIM YM, et al. Upregulation of CXCR4 is functionally crucial for maintenance of stemness in drug-resistant non-small cell lung cancer cells. Oncogene. 2013;32(2):209-221. [41] LUONGO F, COLONNA F, CALAPÀ F, et al. PTEN Tumor-Suppressor: The Dam of Stemness in Cancer. Cancers (Basel). 2019;11(8):1076. |
| [1] | Wang Dandan, Yu Yabin, Liu Shiqi, Yan Yulou, Zhang Jianhuai. Role of long non-coding RNA nuclear-enriched abundant transcript 1 in the differentiation of human umbilical cord mesenchymal stem cells into hepatocytes [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(30): 4847-4851. |
| [2] | Zou Mingming, Ni Li, Zhou Liyu, Li Di, Saijilafu, Wei Shanwen, Zhang Pengfei. Optimization of conditions for intrauterine electroporation transfection in neural stem cells from the subependymal region of fetal mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(30): 4879-4883. |
| [3] | Mi Jianguo, Qiao Rongqin, Liu Shaojin. Bushen Jianpi Huoxue Recipe improves bone metabolism, oxidative stress, and autophagy in osteoporotic rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(26): 4147-4152. |
| [4] | Sun Jinpeng, Liu Jun, Bai Yunfeng, Hua Feng, Wang Haoran, Zheng Hongrui, Wu Tao. Effects of suppressor of cytokine signaling 3 on osteogenic activity in the cartilage of adolescent idiopathic scoliosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(26): 4160-4165. |
| [5] | Cai Zhiguo, Du Shasha, Yang Kun, Zhao Na, Liu Qi. Lipopolysaccharides mediate autophagy of mouse insulinoma βtc6 cells in high glucose state [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(20): 3127-3132. |
| [6] | Tang Mi, Qin Zhen, Wei Zhengxin, Ni Ming, Chai Xiaokang. Effect of ATR gene knockdown on senescence and function of rat bone marrow endothelial progenitor cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(19): 2985-2990. |
| [7] | Zhang Luwen, Dai Pengxiu, Zhang Yihua, Chen Yijing, Wang Jinglu, Li Jiakai. SV40 large T gene induces immortalization of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(13): 1969-1973. |
| [8] | Nie Huijuan, Huang Zhichun. The role of Hedgehog signaling pathway in transforming growth factor beta1-induced myofibroblast transdifferentiation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 754-760. |
| [9] | Wang Xiang, Wei Chaojun, Wang Yao, Pan Yujia, Liu Danan. Lentiviral vector overexpressing FNDC5 inhibits proliferation and migration of smooth muscle cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(35): 5670-5675. |
| [10] | Zheng Weipeng, Hu Weijian, Zhao Guoyuan, Wei Hewei, Liu Zhijun, Wan Lei, Chen Sheng, Liao Zhihao. Effects of Bushen Jianpi Huoxue Recipe on cell proliferation and alkaline phosphatase activity in Beclin-1 overexpressing and silencing MC-3T3-E1 cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(29): 4650-4655. |
| [11] | Zheng Feng, Zhang Fucai, Xu Zhe. MicroRNA-98-5p promotes osteoblast proliferation and differentiation: possibilities and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(26): 4112-4117. |
| [12] | Lu Yuyun, Huang Mei, Shi Xinlei, Chen Baoyan. Bibliometric and visualization analysis of breast cancer stem cell literature from 2011 to 2020 based on Web of Science database [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 4001-4008. |
| [13] | Xu Guilan, Song Jiansheng, Yang Shijiang. Regulation of S100A4 gene silencing by ultrasound microbubbles on the stemness and epithelial-mesenchymal transformation of gastric cancer stem cells#br# [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 4025-4031. |
| [14] | Chen Xinling, Wang Shenglan. Cell autophagy, pathway, regulation and its multiple correlations with pulmonary hypertension [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(2): 311-316. |
| [15] | Dai Yaling, Chen Lewen, He Xiaojun, Lin Huawei, Jia Weiwei, Chen Lidian, Tao Jing, Liu Weilin. Construction of miR-146b overexpression lentiviral vector and the effect on the proliferation of hippocampal neural stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 3024-3030. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||