Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (6): 883-889.doi: 10.12307/2023.228
Previous Articles Next Articles
Spatiotemporal dynamic changes of ependymal cells after spinal cord injury in transgenic mice
Hao Liufang1, Duan Hongmei1, Wang Zijue1, Hao Fei2, Hao Peng1, Zhao Wen1, Gao Yudan1, Yang Zhaoyang1, Li Xiaoguang1
- 1Department of Neurobiology, Captial Medical University, Beijing 100069, China; 2Beijing Advanced Innovation Center for Biomedical Engineering, Medical-Engineering Cross-Innovation Research Institute, Beihang University, Beijing 100191, China
-
Received:2022-01-19Accepted:2022-03-07Online:2023-02-28Published:2022-08-11 -
Contact:Li Xiaoguang, MD, Professor, Department of Neurobiology, Captial Medical University, Beijing 100069, China -
About author:Hao Liufang, Master, Department of Neurobiology, Captial Medical University, Beijing 100069, China -
Supported by:National Natural Science Foundation of China, No. 81941011, 31730030 (to LXG); National Natural Science Foundation of China, No. 31670988, 31971279 (to YZY); National Natural Science Foundation of China, No. 31900749 (to HP); National Natural Science Foundation of China, No. 31771053 (to DHM); National Key Research and Development Program, No. 2017YFC1104002 (to YZY); National Key Research and Development Program, No. 2017YFC1104001 (to LXG); Beijing Science and Technology Program, No. Z181100001818007 (to YZY); Natural Science Foundation Youth Project of Beijing, No. 7214301 (to HF); Natural Science Foundation General Project of Beijing, No. 7222004 (to DHM)
CLC Number:
Cite this article
Hao Liufang, Duan Hongmei, Wang Zijue, Hao Fei, Hao Peng, Zhao Wen, Gao Yudan, Yang Zhaoyang, Li Xiaoguang. Spatiotemporal dynamic changes of ependymal cells after spinal cord injury in transgenic mice[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 883-889.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
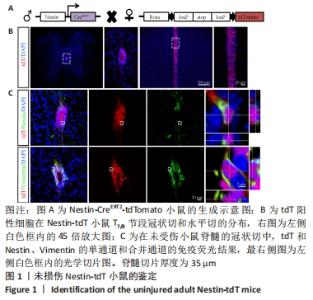
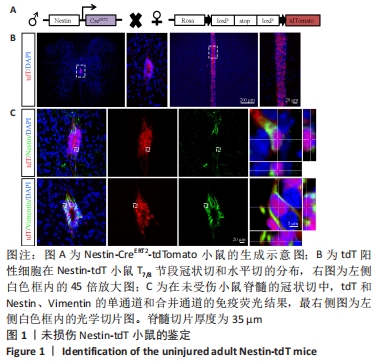
2.1 实验动物数量分析 实验选用小鼠共54只,Nestin-tdT小鼠共36只,其中30只制备了脊髓损伤模型,术中因出血过多死亡1只,其余小鼠在术后恢复良好;Foxj1-tdT小鼠共18只,其中12只制备了脊髓损伤模型,因术后感染死亡1只,其余小鼠在术后恢复良好。共52只小鼠进入结果分析。 2.2 Nestin谱系示踪小鼠的鉴定结果 为靶向室管膜细胞,使用Nestin-CreERT2小鼠与Rosa26-tdTomato(tdT)报告小鼠杂交,获得Nestin-CreERT2-tdTomato小鼠。在他莫昔芬诱导下,Nestin阳性细胞被红色荧光特异性标记。他莫昔芬不注射,红色荧光不表达(数据未显示)。为了确认室管膜细胞表达报告基因,观察了连续注射4 d他莫昔芬,清除7 d后小鼠tdT的表达情况,见图1。脊髓T8节段的冠状切与水平切显示,tdT阳性细胞主要分布在脊髓中央管的室管膜区域,染色结果显示tdT阳性细胞与室管膜相关的标记物中间丝Nestin和波形蛋白(Vimentin,Vim)共标,与以前的文献结果相似[14]。"
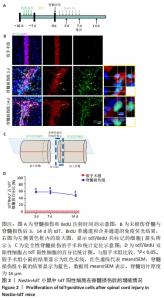
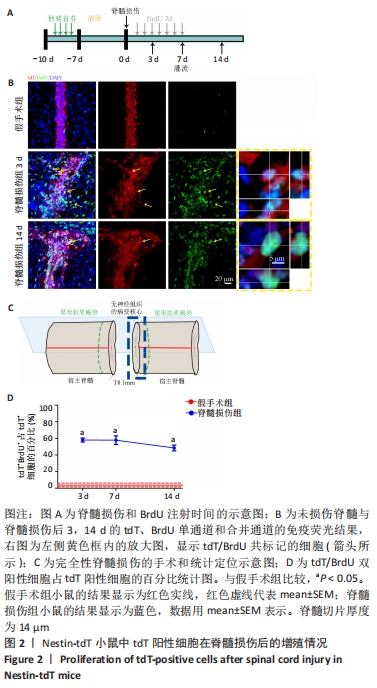
2.3 室管膜细胞在损伤后3 d内大量增殖 为了研究Nestin-tdT小鼠在脊髓损伤后室管膜细胞对损伤的反应,首先观察了未损伤状态下的室管膜细胞,见图2。未损伤时tdT/BrdU双阳性的室管膜细胞只占tdT阳性细胞的(1.2±0.5)%。在脊髓损伤后,tdT阳性细胞在脊髓损伤后3 d内大量增殖,tdT/BrdU双阳性的细胞占tdT阳性细胞总数的(58.1±2.4)%,第3天比第1天,P < 0.05,7-14 d时增殖的室管膜细胞呈下降趋势,7 d时,tdT/BrdU双阳性细胞占tdT阳性细胞总数的百分比为(57.9±5.17)%,14 d时,tdT/BrdU双阳性细胞占比为(48.5±3.4)%,第7天比第3天,P > 0.05;第14天比第7天,P > 0.05。"
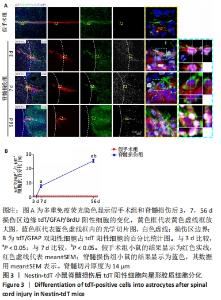
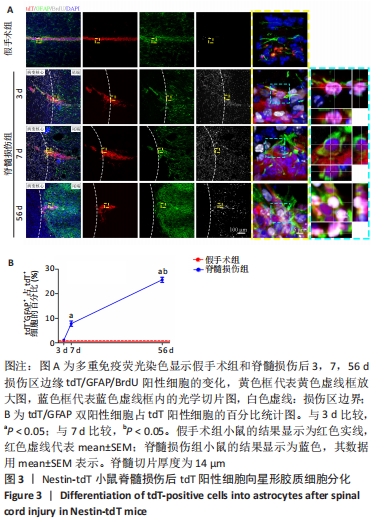
2.4 室管膜在脊髓损伤后分化为星形胶质细胞 为了观察脊髓损伤后室管膜细胞是否可以分化为星形胶质细胞以及它们的时空动态过程,利用免疫荧光染色的方式,GFAP/BrdU标记增殖的星形胶质细胞。未损伤时,靠近室管膜层的星形胶质细胞呈双极形态[15]。损伤边缘附近tdT/GFAP双阳性细胞最早在第3天出现,占比为(1.1±0.1)%,此时室管膜细胞开始离开中央管区域并向损伤部位增殖迁移,第7天时,星形胶质瘢痕边缘有许多长的GFAP阳性星形胶质细胞突起,这些突起相互交织和重叠成致密的网状结构[16],见图3,tdT/GFAP双阳性细胞占tdT阳性细胞总数的百分比为(7.8±1.1)%(第7天比第3天,P < 0.05)。在第56天时,有(25.7±1.0)%的室管膜细胞分化为了星形胶质细胞(第56天与第7天相比,P < 0.05)。"
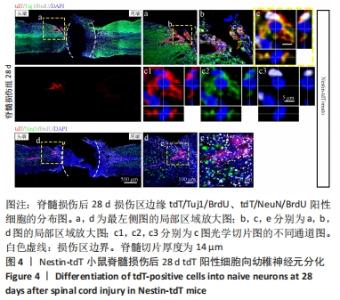
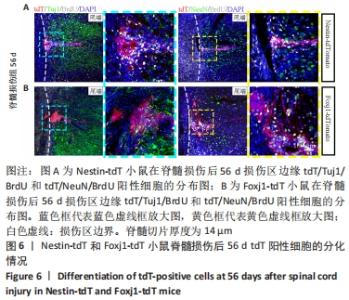
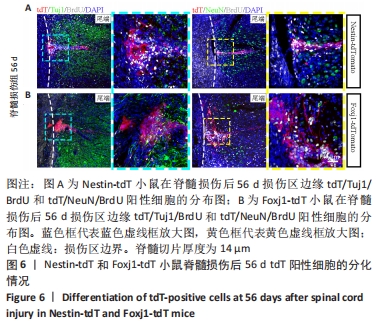
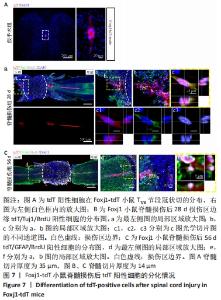
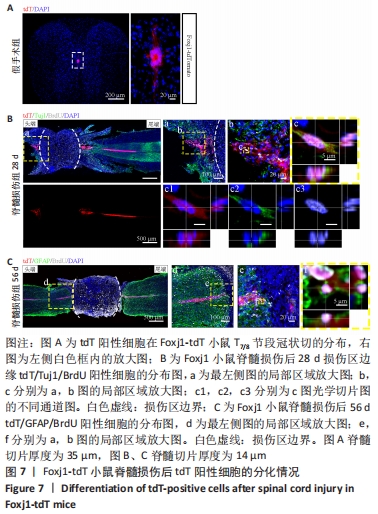
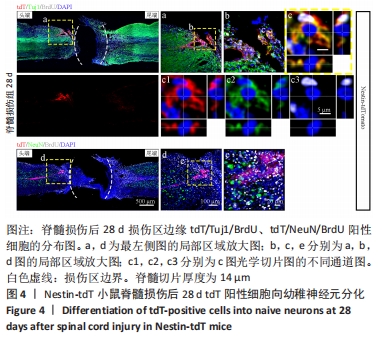
2.5 室管膜细胞可以分化为未成熟的神经元 观察Nestin阳性室管膜细胞在完全横断脊髓损伤后是否可以分化为神经元,借助未成熟神经元的标记物Tuj1和增殖细胞的标记物BrdU免疫荧光双标,判断新生神经元的形成。在未受伤的脊髓中,tdT阳性细胞不表达Tuj1。在脊髓损伤后第28天,病变区边缘出现tdT/Tuj1双阳性细胞,显示部分室管膜细胞分化为未成熟的神经元,见图4。统计学分析显示在病灶边缘200 μm内tdT/Tuj1双阳性细胞占tdT阳性细胞总数的(3.3±0.7)%,见图5。在同一时间点,通过成熟神经元标记物NeuN的免疫荧光染色,判断室管膜细胞向成熟神经元的分化情况,结果显示:在脊髓损伤区及损伤周围并未观察到tdT阳性的室管膜细胞分化为成熟的神经元。在56 d时,在脊髓损伤区及损伤周围并未发现tdT阳性细胞与Tuj1或NeuN共表达,见图6A。 2.6 室管膜细胞短暂的向幼稚神经元分化 Nestin在室管膜细胞的背侧和腹侧表达,Foxj1可以标记几乎所有的室管膜细胞,所以使用了Foxj1-CreERT2-tdT小鼠。启动子Foxj1在室管膜细胞中特异性控制CreER表达,因此更适用于追踪成年小鼠脊髓损伤后室管膜细胞的分化命运[17]。Foxj1-tdT小鼠在手术前连续注射他莫昔芬4 d,以激活可诱导的Cre重组酶并启动报告基因的转录。在脊髓损伤后第28天,观察到在tdT阳性细胞中只有(3.2±0.3)%表达未成熟神经元的标记物Tuj1,见图5,图7(与Nestin-tdT小鼠脊髓损伤后28 d相比,P > 0.05)。在第56天时tdT/GFAP双阳性的星形胶质细胞占tdT阳性室管膜细胞的(12.3±1.0)%。免疫荧光结果显示:在脊髓损伤第56天时,tdT没有与Tuj1或NeuN共表达,见图6B。"
| [1] LEE-LIU D, EDWARDS-FARET G, TAPIA VS, et al. Spinal cord regeneration: lessons for mammals from non-mammalian vertebrates. Genesis. 2013; 51(8):529-544. [2] LI X, FLORIDDIA EM, TOSKAS K, et al. Regenerative Potential of Ependymal Cells for Spinal Cord Injuries Over Time. EBioMedicine. 2016;13:55-65. [3] MELETIS K, BARNABÉ-HEIDER F, CARLÉN M, et al. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008; 6(7):e182. [4] LLORENS-BOBADILLA E, CHELL JM, LE MERRE P, et al. A latent lineage potential in resident neural stem cells enables spinal cord repair. Science. 2020;370(6512):eabb8795. [5] 段红梅, 宋伟, 赵文, 等. 成年哺乳类脊髓损伤后的内源性神经发生[J]. 中国科学:生命科学,2016,46(12):1382-1387. [6] BRANDA CS, DYMECKI SM. Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice. Dev Cell. 2004;6(1):7-28. [7] MAO J, BARROW J, MCMAHON J, et al. An ES cell system for rapid, spatial and temporal analysis of gene function in vitro and in vivo. Nucleic Acids Res. 2005;33(18):e155. [8] METZGER D, CHAMBON P. Site- and time-specific gene targeting in the mouse. Methods. 2001;24(1):71-80. [9] SANDLESH P, JUANG T, SAFINA A, et al. Uncovering the fine print of the CreERT2-LoxP system while generating a conditional knockout mouse model of Ssrp1 gene. PLoS One. 2018;13(6):e0199785. [10] HAYASHI S, MCMAHON AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244(2):305-318. [11] CARLÉN M, MELETIS K, BARNABÉ-HEIDER F, et al. Genetic visualization of neurogenesis. Exp Cell Res. 2006;312(15):2851-2859. [12] FRISÉN J, JOHANSSON CB, TÖRÖK C, et al. Rapid, widespread, and longlasting induction of nestin contributes to the generation of glial scar tissue after CNS injury. J Cell Biol. 1995;131(2):453-464. [13] MUTHUSAMY N, VIJAYAKUMAR A, CHENG G JR, et al. A knock-in Foxj1(CreERT2::GFP) mouse for recombination in epithelial cells with motile cilia. Genesis. 2014;52(4):350-358. [14] BERNAL A, ARRANZ L. Nestin-expressing progenitor cells: function, identity and therapeutic implications. Cell Mol Life Sci. 2018;75(12): 2177-2195. [15] BARNABÉ-HEIDER F, GÖRITZ C, SABELSTRÖM H, et al. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell. 2010; 7(4):470-482. [16] WANNER IB, ANDERSON MA, SONG B, et al. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci. 2013;33(31):12870-12886. [17] LI X, FLORIDDIA EM, TOSKAS K, et al. FoxJ1 regulates spinal cord development and is required for the maintenance of spinal cord stem cell potential. Exp Cell Res. 2018;368(1):84-100. [18] DERVAN AG, ROBERTS BL. Reaction of spinal cord central canal cells to cord transection and their contribution to cord regeneration. J Comp Neurol. 2003;458(3):293-306. [19] SABELSTRÖM H, STENUDD M, FRISÉN J. Neural stem cells in the adult spinal cord. Exp Neurol. 2014;260:44-49. [20] YU SS, LI ZY, XU XZ, et al. M1-type microglia can induce astrocytes to deposit chondroitin sulfate proteoglycan after spinal cord injury. Neural Regen Res. 2022;17(5):1072-1079. [21] CREGG JM, DEPAUL MA, FILOUS AR, et al. Functional regeneration beyond the glial scar. Exp Neurol. 2014;253:197-207. [22] BUSCH SA, SILVER J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17(1):120-127. [23] KAWAGUCHI A, MIYATA T, SAWAMOTO K, et al. Nestin-EGFP transgenic mice: visualization of the self-renewal and multipotency of CNS stem cells. Mol Cell Neurosci. 2001;17(2):259-273. [24] DULIN JN, ADLER AF, KUMAMARU H, et al. Injured adult motor and sensory axons regenerate into appropriate organotypic domains of neural progenitor grafts. Nat Commun. 2018;9(1):84. [25] YANG Z, ZHANG A, DUAN H, et al. NT3-chitosan elicits robust endogenous neurogenesis to enable functional recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2015;112(43):13354-13359. [26] WALKER CL, FRY CME, WANG J, et al. Functional and Histological Gender Comparison of Age-Matched Rats after Moderate Thoracic Contusive Spinal Cord Injury. J Neurotrauma. 2019;36(12):1974-1984. [27] FUKUTOKU T, KUMAGAI G, FUJITA T, et al. Sex-Related Differences in Anxiety and Functional Recovery after Spinal Cord Injury in Mice. J Neurotrauma. 2020;37(21):2235-2243. [28] BAINE RE, JOHNSTON DT, STRAIN MM, et al. Noxious Stimulation Induces Acute Hemorrhage and Impairs Long-Term Recovery after Spinal Cord Injury (SCI) in Female Rats: Evidence Estrous Cycle May Have a Modulatory Effect. Neurotrauma Rep. 2022;3(1):70-86. [29] REN Y, AO Y, O’SHEA TM, et al. Ependymal cell contribution to scar formation after spinal cord injury is minimal, local and dependent on direct ependymal injury. Sci Rep. 2017;7:41122. [30] SABELSTRÖM H, STENUDD M, RÉU P, et al. Resident neural stem cells restrict tissue damage and neuronal loss after spinal cord injury in mice. Science. 2013;342(6158):637-640. [31] MOTHE AJ, TATOR CH. Proliferation, migration, and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult rat. Neuroscience. 2005;131(1):177-187. [32] HU J, JIN LQ, SELZER ME. Inhibition of central axon regeneration: perspective from chondroitin sulfate proteoglycans in lamprey spinal cord injury. Neural Regen Res. 2022;17(9):1955-1956. [33] ANDERSON MA, BURDA JE, REN Y, et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532(7598): 195-200. [34] LU P, KADOYA K, TUSZYNSKI MH. Axonal growth and connectivity from neural stem cell grafts in models of spinal cord injury. Curr Opin Neurobiol. 2014;27:103-109. [35] MA D, ZHAO Y, HUANG L, et al. A novel hydrogel-based treatment for complete transection spinal cord injury repair is driven by microglia/macrophages repopulation. Biomaterials. 2020;237:119830. [36] YANG Z, DUAN H, MO L, et al. The effect of the dosage of NT-3/chitosan carriers on the proliferation and differentiation of neural stem cells. Biomaterials. 2010;31(18):4846-4854. [37] NORISTANI HN, SABOURIN JC, BOUKHADDAOUI H, et al. Spinal cord injury induces astroglial conversion towards neuronal lineage. Mol Neurodegener. 2016;11(1):68. [38] GUO Y, LIU S, ZHANG X, et al. Sox11 promotes endogenous neurogenesis and locomotor recovery in mice spinal cord injury. Biochem Biophys Res Commun. 2014;446(4):830-835. [39] YANG L, LI G, YE J, et al. Substance P enhances endogenous neurogenesis to improve functional recovery after spinal cord injury. Int J Biochem Cell Biol. 2017;89:110-119. |
| [1] | Yang Jiujie, Li Zhi, Wang Shujie, Tian Ye, Zhao Wei. Intraoperative neurophysiological monitoring of functional changes following durotomy with decompression for acute spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1232-1236. |
| [2] | Yang Zhishan, Tang Zhenglong. YAP/TAZ, a core factor of the Hippo signaling pathway, is involved in bone formation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1264-1271. |
| [3] | Gao Yu, Han Jiahui, Ge Xin. Immunoinflammatory microenvironment after spinal cord ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1300-1305. |
| [4] | Liu Xiaolin, Mu Xinyue, Ma Ziyu, Liu Shutai, Wang Wenlong, Han Xiaoqian, Dong Zhiheng. Effect of hydrogel-loaded simvastatin microspheres on osteoblast proliferation and differentiation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 998-1003. |
| [5] | Liu Wentao, Feng Xingchao, Yang Yi, Bai Shengbin. Effect of M2 macrophage-derived exosomes on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 840-845. |
| [6] | Long Yanming, Xie Mengsheng, Huang Jiajie, Xue Wenli, Rong Hui, Li Xiaojie. Casein kinase 2-interaction protein-1 regulates the osteogenic ability of bone marrow mesenchymal stem cells in osteoporosis rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 878-882. |
| [7] | Li Xinyue, Li Xiheng, Mao Tianjiao, Tang Liang, Li Jiang. Three-dimensional culture affects morphology, activity and osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 846-852. |
| [8] | Yuan Wei, Liu Jingdong, Xu Guanghui, Kang Jian, Li Fuping, Wang Yingjie, Zhi Zhongzheng, Li Guanwu. Osteogenic differentiation of human perivascular stem cells and its regulation based on Wnt/beta-catenin signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 866-871. |
| [9] | Qiao Luhui, Ma Ziyu, Guo Haoyu, Hou Yudong. Comparison of puerarin and icariin on the biological properties of mouse preosteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 872-877. |
| [10] | Liao Yidong, Ming Jiang, Song Wenxue, Wang Zili, Zhang Yu, Liao Yifei, Xu Kaya, Yang Hua. An experimental method for simultaneously culturing primary cortical and hippocampal neurons [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 897-902. |
| [11] | Li Xiaoyin, Yang Xiaoqing, Chen Shulian, Li Zhengchao, Wang Ziqi, Song Zhen, Zhu Daren, Chen Xuyi. Collagen/silk fibroin scaffold combined with neural stem cells in the treatment of traumatic spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 890-896. |
| [12] | Zhang Qijian, Xu Ximing. Acquisition and application of ectodermal mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 928-934. |
| [13] | Zhao Siqi, Du Juan, Qu Haifeng, Li Jianmin, Zhang Yuxin, Liu Junjie. Effects of enriched environment combined with melatonin on learning and memory function and brain neuron apoptosis in SAMP8 mice [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 701-706. |
| [14] | Chen Guodong, Zheng Meiyan, Zhang Peng, Wang Zhenchao, Jin Lixin. Changes in sensory neurons and astrocytes and the expression of interleukin 1beta and glial fibrillary acidic protein in the rat spinal cord after selective dorsal rhizotomy [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 726-731. |
| [15] | Li Long, Li Guangdi, Shi Hao, Deng Keqi. Circular RNA as a competing endogenous RNA is involved in the regulation of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 751-757. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||