Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (34): 5448-5454.doi: 10.12307/2023.839
Previous Articles Next Articles
Preparation of cartilage decellularized extracellular matrix-loaded composite nanofiber scaffolds based on two-nozzle electrospinning
Teng Jianxiang1, Zhu Jisheng1, Yuan Daizhu2, Wang Zhen1, Zhou Yuhu2, Tian Xiaobin1, 2
- 1Guizhou Medical University, Guiyang 550001, Guizhou Province, China; 2Department of Orthopedics, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China
-
Received:2022-11-02Accepted:2022-12-15Online:2023-12-08Published:2023-04-20 -
Contact:Tian Xiaobin, Chief physician, Guizhou Medical University, Guiyang 550001, Guizhou Province, China; Department of Orthopedics, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China -
About author:Teng Jianxiang, Master candidate, Physician, Guizhou Medical University, Guiyang 550001, Guizhou Province, China -
Supported by:Guizhou Science and Technology Plan Project, No. [2021]072 (to TXB)
CLC Number:
Cite this article
Teng Jianxiang, Zhu Jisheng, Yuan Daizhu, Wang Zhen, Zhou Yuhu, Tian Xiaobin. Preparation of cartilage decellularized extracellular matrix-loaded composite nanofiber scaffolds based on two-nozzle electrospinning[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5448-5454.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
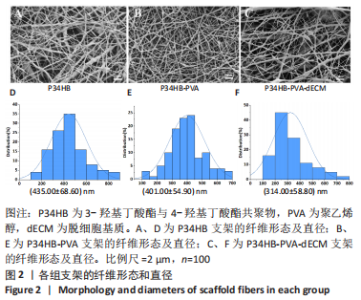
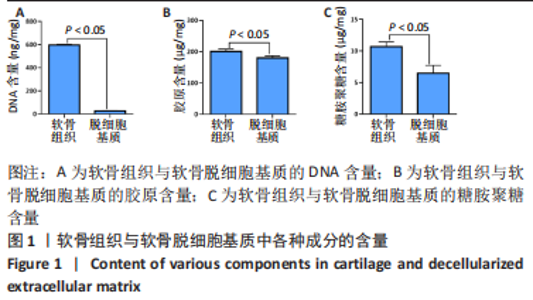
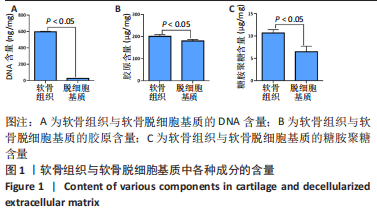
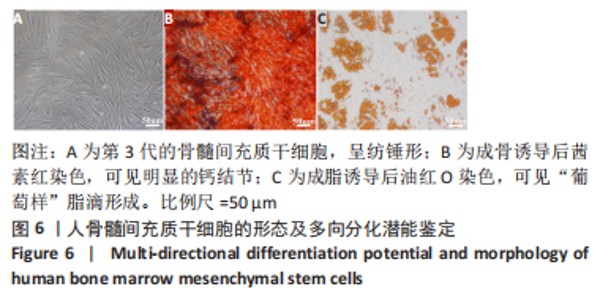
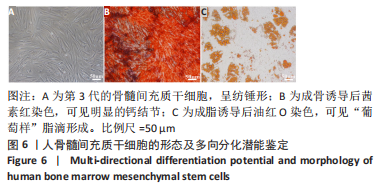
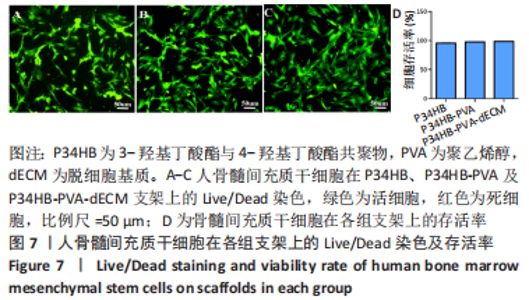
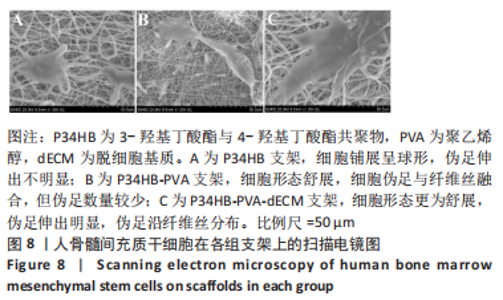
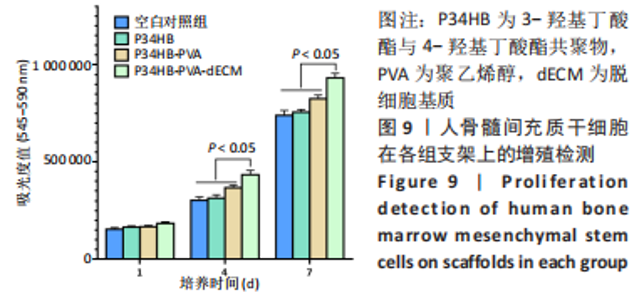
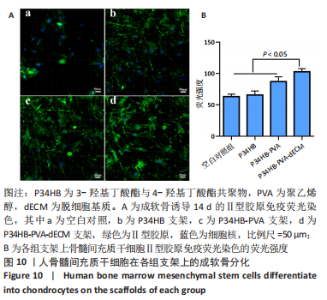
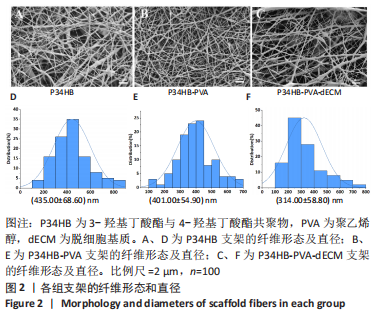
经过酶、化学及超声振荡洗涤处理后,所制备的软骨dECM中残余的DNA含量为(25.53±2.06) ng/mg,较正常软骨组织DNA含量(595.83±6.35) ng/mg明显减少(P < 0.05 ),去除DNA含量约95%。dECM中的胶原含量(179.53±7.20) μg/mg,较软骨组织中的胶原含量(200.43±7.78) μg/mg降低(P < 0.05),保留了胶原约90%;dECM中的糖胺聚糖含量为(6.48±1.23) μg/mg,较正常软骨组织的糖胺聚糖含量(10.69±0.74) μg/mg降低(P < 0.05),保留了糖胺聚糖约60%。 2.2 各组支架物理性能表征结果 2.2.1 各组支架形态学观察 各组支架扫描电镜观察结果如图2所示,各组支架纤维呈随机分布,纤维之间互相连接呈现多孔结构。通过测量各组支架的纤维直径,P34HB支架的纤维直径为(435.00±68.60) nm,P34HB-PVA支架纤维直径为(401.00±54.90) nm,P34HB-PVA-dECM支架的纤维直径为(314.00±58.80) nm。"
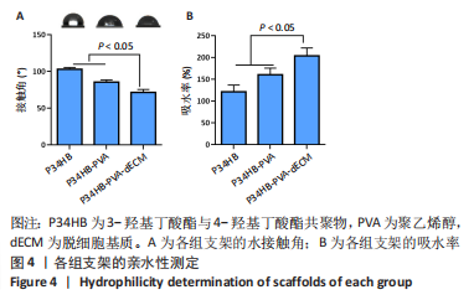
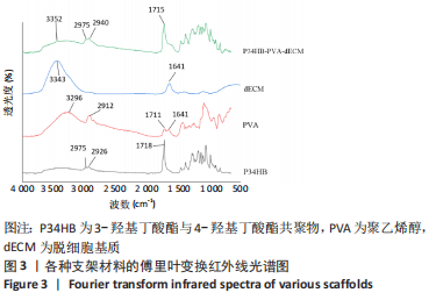
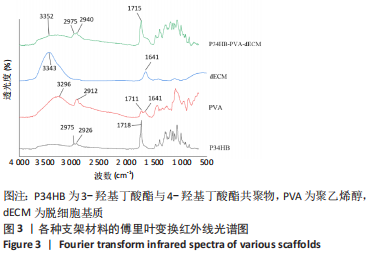
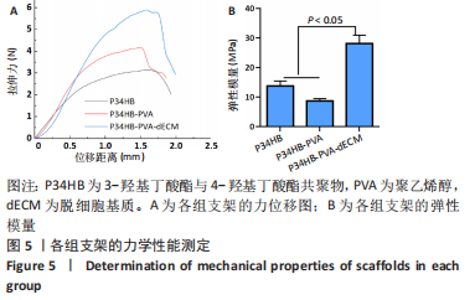
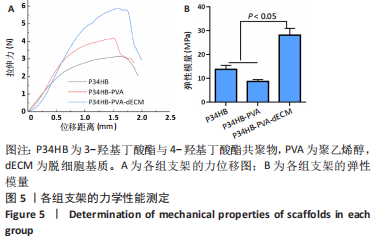
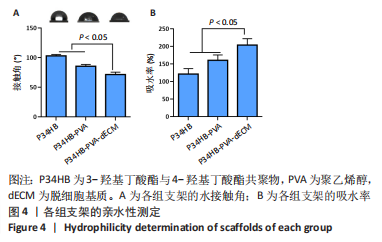
2.2.3 各组支架亲水性能检测结果 P34HB、P34HB-PVA、P34HB-PVA-dECM支架的接触角分别为(102.91±2.21)°,(85.44±2.96)°,(71.42±4.01)°;与其他组相比,P34HB-PVA-dECM支架的水接触角最小(P < 0.05),见图4A。各组支架的吸水率结果如图4B所示,P34HB、P34HB-PVA、P34HB-PVA-dEC支架的吸水率分别为(121.04±15.49)%,(160.38±15.20)%,(203.75±17.99)%,P34HB-PVA-dECM支架的吸水率最高(P < 0.05),说明P34HB-PVA-dECM支架的亲水性最好。"
| [1] ZHANG FX, LIU P, DING W, et al. Injectable mussel-Inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomaterials. 2021;278:121169. [2] WANG S, YANG L, CAI B, et al. Injectable hybrid inorganic nanoscaffold as rapid stem cell assembly template for cartilage repair. Natl Sci Rev. 2022; 9(4):nwac037. [3] AL-QURAYSHI Z, WAFA EI, ROSSI MEYER MK, et al. Tissue engineering the pinna: comparison and characterization of human decellularized auricular biological scaffolds. ACS Appl Bio Mater. 2021;4(9):7234-7242. [4] ORTH P, GAO L, MADRY H. Microfracture for cartilage repair in the knee: a systematic review of the contemporary literature. Knee Surg Sport Traumatol Arthrosc. 2020;28(3):670-706. [5] TIAN G, JIANG S, LI J, et al. Cell-free decellularized cartilage extracellular matrix scaffolds combined with interleukin 4 promote osteochondral repair through immunomodulatory macrophages: in vitro and in vivo preclinical study. Acta Biomater. 2021;127:131-145. [6] BLUM JC, SCHENCK TL, BIRT A, et al. Artificial decellularized extracellular matrix improves the regenerative capacity of adipose tissue derived stem cells on 3D printed polycaprolactone scaffolds. J Tissue Eng. 2021;12: 20417314211022242. [7] 胡秋羽,杨龙,杨勇,等.聚己二酸丁二醇酯-对苯二甲酸丁二醇酯/Ⅰ型胶原取向纤维促进前交叉韧带断裂后腱-骨愈合[J].中国组织工程研究,2022,26(27):4314-4319. [8] XUE J, WU T, DAI Y, et al. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem Rev. 2019;119(8):5298-5415. [9] BARHOUM A, PAL K, RAHIER H, et al. Nanofibers as new-generation materials: from spinning and nano-spinning fabrication techniques to emerging applications. Appl Mater Today. 2019;(17):1-35. [10] KANIUK Ł, STACHEWICZ U. Development and advantages of biodegradable PHA polymers based on electrospun PHBV fibers for tissue engineering and other biomedical applications. ACS Biomater Sci Eng. 2021;7(12):5339-5362. [11] BASNETT P, MATHARU RK, TAYLOR CS, et al. Harnessing polyhydroxyalkanoates and pressurized gyration for hard and soft tissue engineering. ACS Appl Mater Interfaces. 2021;13:32624-32639. [12] YE C, HU P, MA MX, et al. PHB/PHBHHx scaffolds and human adipose-derived stem cells for cartilage tissue engineering. Biomaterials. 2009;30(26):4401-4406. [13] 刘鋆,杨龙,王伟宇,等.聚 3-羟基丁酸酯 4-羟基丁酸酯/聚乙二醇/氧化石墨烯组织工程支架的制备和性能评价[J].中国组织工程研究,2021,25(22):3466-3472. [14] MA MX, LIU Q, YE C, et al. Preparation of P3HB4HB/(Gelatin + PVA) composite scaffolds by coaxial electrospinning and its biocompatibility evaluation. Biomed Res Int. 2017;(2017):9251806. [15] MONZAVI SM, KAJBAFZADEH AM, SABETKISH S, et al. Extracellular matrix scaffold using decellularized Cartilage for hyaline cartilage regeneration. Adv Exp Med Biol. 2021;1345:209-223. [16] CHEN M, FENG Z, GUO W, et al. PCL-MECM- Based hydrogel hybrid scaffolds and meniscal fibrochondrocytes promote whole meniscus regeneration in a rabbit meniscectomy model. ACS Appl Mater Interfaces. 2019;11(44): 41626-41639. [17] IRANI M, NODEH SM. PVA/kappa-carrageenan/Au/camptothecin/pegylated-polyurethane/paclitaxel nanofibers against lung cancer treatment. RSC Adv. 2022;12(25):16310-16318. [18] YANG L, ZHAO Y, CUI DB, et al. Coaxial bioelectrospinning of P34HB/PVA microfibers biomimetic scaffolds with simultaneity cell-laden for improving bone regeneration. Mater Design. 2022;213:110349. [19] 赵超尘.脱细胞基质覆层壳聚糖纤维载体的制备和生物学评价[D].广州:南方医科大学,2020. [20] LIU HW, SU WT, LIU CY, et al. Highly organized porous gelatin-based scaffold by microfluidic 3D-foaming technology and dynamic culture for cartilage tissue engineering. Int J Mol Sci. 2022;23(15):8449. [21] XI Y, GE J, GUO Y, et al. Biomimetic elastomeric polypeptidebased nanofibrous matrix for overcoming multidrug-resistant bacteria and enhancing full-thickness wound healing/skin regeneration. ACS Nano. 2018; 12:10772-10784. [22] ZELINKA A, ROELOFS AJ, KANDEL RA, et al. Cellular therapy and tissue engineering for cartilage repair. Osteoarthritis Cartilage. 2022;30(12): 1547-1560. [23] ZHANG Q, NETTLESHIP I, SCHMELZER E, et al. Tissue engineering and regenerative medicine therapies for cell senescence in bone and cartilage. Tissue Eng Part B Rev. 2020;26(1):64-78. [24] KIM HS, MANDAKHBAYAR N, KIM HW, et al. Protein-reactive nanofibrils decorated with cartilage-derived decellularized extracellular matrix for osteochondral defects. Biomaterials. 2021;269:120214. [25] HONG J, YEO M, YANG GH, et al. Cell-electrospinning and its application for tissue engineering. Int J Mol Sci. 2019;20:6208. [26] XING H, LEE H, LUO L, et al. Extracellular matrix-derived biomaterials in engineering cell function. Biotechnol Adv. 2020;42:107421. [27] FIORDALISI M, SILVA AJ, BARBOSA M, et al. Decellularized scaffolds for intervertebral disc regeneration. Trends Biotechnol. 2020;38(9):947-951. [28] GAO S, CHEN M, WANG P, et al. An electrospun fiber reinforced scaffold promotes total meniscus regeneration in rabbit meniscectomy model. Acta Biomater. 2018;73:127-140. [29] ZHANG X, CHEN X, HONG H, et al. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact Mater. 2021;23(10):15-31. [30] ROTHRAUFF BB, COLUCCINO L, GOTTARDI R, et al. Efficacy of thermoresponsive, photocrosslinkable hydrogels derived from decellularized tendon and cartilage extracellular matrix for cartilage tissue engineering. J Tissue Eng Regen Med. 2018;12(1):e159-e170. [31] GAO S, GUO W, CHEN M, et al. Fabrication and characterization of electrospun nanofibers composed of decellularized meniscus extracellular matrix and polycaprolactone for meniscus tissue engineering. J Mater Chem B. 2017;5(12):2273-2285. [32] MISZUK JM, XU T, YAO Q, et al. Functionalization of PCL-3D electrospun nanofibrous scaffolds for improved BMP2-induced bone formation. Appl Mater Today. 2018;10:194-202. [33] 蒲丛丛,何建新,崔世忠,等.电场对双喷头喷气静电纺纳米纤维成形的影响[J].材料导报,2013,27(7):86-89. [34] 任浩征,潘超,许迎,等.ZnO 表面改性 Zn-Cu 组织工程支架的研究[J].表面技术,2021,50(2):58-65. [35] LI X, LIU M, CHEN F, et al. Design of hydroxyapatite bioceramics with micro-/nano-topographies to regulate the osteogenic activities of bone morphogenetic protein-2 and bone marrow stromal cells. Nanoscale. 2020; 12(13):7284-7300. [36] GIRAO AF, SEMITELA A, PEREIRA AL, et al. Microfabrication of a biomimetic arcade-like electrospun scaffold for cartilage tissue engineering applications. J Mater Sci Mater Med. 2020;31(8):69. [37] GRESHAM RCH, BAHNEY CS, LEACH JK. Growth factor delivery using extracellular matrix-mimicking substrates for musculoskeletal tissue engineering and repair. Bioact Mater. 2021;6(7):1945-1956. [38] ZHU W, CAO L, SONG C, et al. Cell-derived decellularized extracellular matrix scaffolds for articular cartilage repair. Int J Artif Organs. 2021;44(4):269-281. [39] SHAHVERDI F, BARATI A, SALEHI E, et al. Biaxial electrospun nanofibers based on chitosan-poly (vinyl alcohol) and poly (Ɛ-caprolactone) modified with CeAlO3 nanoparticles as potential wound dressing materials. Int J Biol Macromol. 2022;221:736-750. [40] PEDRAM RAD Z, MOKHTARI J, ABBASI M, et al. Calendula officinalis extract/PCL/Zein/Gum arabic nanofibrous bio-composite scaffolds via suspension, two-nozzle and multilayer electrospinning for skin tissue engineering. Int J Biol Macromol. 2019;135:530-543. |
| [1] | Sun Kexin, Zeng Jinshi, Li Jia, Jiang Haiyue, Liu Xia. Mechanical stimulation enhances matrix formation of three-dimensional bioprinted cartilage constructs [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(在线): 1-7. |
| [2] | Huang Linke, Wei Linhua, Jiang Jie, Liu Qian, Chen Weiwei. Effects of estrogen combined with treadmill exercise on bone mass and articular cartilage in ovariectomized mice [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1166-1171. |
| [3] | Xu Xingxing, Wen Chaoju, Meng Maohua, Wang Qinying, Chen Jingqiao, Dong Qiang. Carbon nanomaterials in oral implant [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1062-1070. |
| [4] | Yang Yitian, Wang Lu, Yao Wei, Zhao Bin. Application of the interaction between biological scaffolds and macrophages in bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1071-1079. |
| [5] | Li Cheng, Zheng Guoshuang, Kuai Xiandong, Yu Weiting. Alginate scaffold in articular cartilage repair [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1080-1088. |
| [6] | Xu Cong, Zhao He, Sun Yan. Regeneration of facial nerve injury repaired by biomaterial nerve conduits [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1089-1095. |
| [7] | Chen Shisong, Liu Xiaohong, Xu Zhiyun. Current status and prospects of bioprosthetic heart valves [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1096-1102. |
| [8] | Shi Yehong, Wang Cheng, Chen Shijiu. Early thrombosis and prevention of small-diameter blood vessel prosthesis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1110-1116. |
| [9] | Tang Haotian, Liao Rongdong, Tian Jing. Application and design of piezoelectric materials for bone defect repair [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1117-1125. |
| [10] | Xu Yan, Li Ping, Lai Chunhua, Zhu Peijun, Yang Shuo, Xu Shulan. Piezoelectric materials for vascularized bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1126-1132. |
| [11] | Li Xinyue, Li Xiheng, Mao Tianjiao, Tang Liang, Li Jiang. Three-dimensional culture affects morphology, activity and osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 846-852. |
| [12] | Li Xiaoyin, Yang Xiaoqing, Chen Shulian, Li Zhengchao, Wang Ziqi, Song Zhen, Zhu Daren, Chen Xuyi. Collagen/silk fibroin scaffold combined with neural stem cells in the treatment of traumatic spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 890-896. |
| [13] | Yuan Bo, Xie Lide, Fu Xiumei. Schwann cell-derived exosomes promote the repair and regeneration of injured peripheral nerves [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 935-940. |
| [14] | Qin Yuxing, Ren Qiangui, Li Zilong, Quan Jiaxing, Shen Peifeng, Sun Tao, Wang Haoyu. Action mechanism and prospect of bone microvascular endothelial cells for treating femoral head necrosis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 955-961. |
| [15] | Xiong Bohan, Yu Yang, Lu Xiaojun, Wang Xu, Yang Tengyun, Zhang Yaozhang, Liao Xinyu, Zhou Xiaoxiang, He Lu, Li Yanlin. Research progress in promoting tendon to bone healing during anterior cruciate ligament reconstruction [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 779-786. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||