| [1] De Angelis CD, Drazen JM, Frizelle FA, et al.Is this clinical trial fully registered?--A statement from the International Committee of Medical Journal Editors.N Engl J Med.2005; 352(23):2436-2438.
[2] 晏小勇,陈永法.美国临床试验注册与结果公开制度研究[J].中国新药杂志,2011,20(7):577-581.
[3] 高雪山,钟紫红.实行临床试验注册减少医学期刊发表偏倚[J].中国科技期刊研究,2009,20(5):854-857.
[4] 李昕雪,韩梅,王禹毅,等.临床试验的国际注册及在美国临床试验注册平台注册的意义与方法[J].中医杂志,2013,54(19): 1640-1643.
[5] Abaid LN, Grimes DA, Schulz KF. Reducing publication bias of prospective clinical trials through trial registration. Contraception. 2007;76(5):339-341.
[6] Bonita RE, Adams S, Whellan DJ. Reporting of clinical trials: publication, authorship, and trial registration. Heart Fail Clin. 2011;7(4):561-567.
[7] 严佳,裴建.临床试验注册在针灸临床的应用[J].中华针灸电子杂志,2014,3(1):8-12.
[8] 李晓婷,张晓鹏,李艳玲,等.我国影像临床试验注册情况及其对科研管理的启示[J].中国医院管理,2013,33(9):62-64.
[9] 曹莹莹,林能明,方罗.中国临床试验注册中心在册药物临床试验分析[J].中国药房,2012,23(41):3858-3860.
[10] Lin HT, Kao CL, Lee KH, et al.Enhancement of insulin-producing cell differentiation from embryonic stem cells using pax4-nucleofection method.World J Gastroenterol. 2007;13(11):1672-1679.
[11] Vaca P, Martín F, Vegara-Meseguer JM, et al.Induction of differentiation of embryonic stem cells into insulin-secreting cells by fetal soluble factors.Stem Cells.2006;24(2):258-265.
[12] Nardo B, Masetti M, Urbani L, et al.Liver transplantation from donors aged 80 years and over: pushing the limit.Am J Transplant.2004;4(7):1139-1147.
[13] Tang DQ, Wang Q, Burkhardt BR, et al.In vitro generation of functional insulin-producing cells from human bone marrow-derived stem cells, but long-term culture running risk of malignant transformation.Am J Stem Cells. 2012;1(2): 114-127.
[14] Gao F, Wu DQ, Hu YH, et al.Extracellular matrix gel is necessary for in vitro cultivation of insulin producing cells from human umbilical cord blood derived mesenchymal stem cells. Chin Med J (Engl). 2008;121(9):811-818.
[15] Ngoc PK, Phuc PV, Nhung TH, et al.Improving the efficacy of type 1 diabetes therapy by transplantation of immunoisolated insulin-producing cells.Hum Cell.2011;24(2):86-95.
[16] Gang EJ, Hong SH, Jeong JA, et al.In vitro mesengenic potential of human umbilical cord blood-derived mesenchymal stem cells.Biochem Biophys Res Commun. 2004; 321(1):102-108.
[17] Ito T, Itakura S, Todorov I, et al.Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function.Transplantation.2010;89(12):1438-1445.
[18] Fleischmann E, Marschalek C, Schlemitz K, et al.Nitrous oxide may not increase the risk of cancer recurrence after colorectal surgery: a follow-up of a randomized controlled trial.BMC Anesthesiol. 2009;9:1.
[19] Voils CI, Yancy WS Jr, Kovac S, et al. Study protocol: Couples Partnering for Lipid Enhancing Strategies (CouPLES) - a randomized, controlled trial. Trials. 2009;10:10.
[20] Cima R, Joore M, Maes I, et al.Cost-effectiveness of multidisciplinary management of Tinnitus at a specialized Tinnitus centre.BMC Health Serv Res. 2009;9:29.
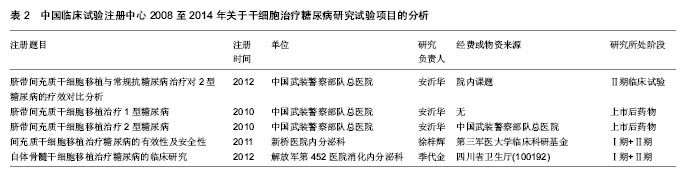
[21] 季代金,黄茂涛.干细胞移植与糖尿病[J].临床军医杂志,2011, 39(1):168-171.
[22] 季代金,黄茂涛,王维萍,等.自体骨髓干细胞治疗2型糖尿病2例[J].临床军医杂志,2011,39(1):199-200.
[23] 姚金萍,段炼,童强,等.自体骨髓干细胞移植治疗2型糖尿病的安全性及有效性[J].第三军医大学学报,2012,34(1):74-77.
[24] 隆敏,郑宏庭,童强,等.自体骨髓干细胞胰腺定向移植治疗糖尿病1例[J].第三军医大学学报,2010,32(6):524.
[25] 郑培,安沂华,王晓东,等.糖尿病周围神经病变神经电生理特点分析[J].解放军医学院学报,2013,34(6):590-592,649.
[26] 郑培,安沂华,王晓东,等.脐带间充质干细胞移植治疗糖尿病周围神经病变的疗效观察[J].武警医学,2013,24(5):398-401.
[27] Liu X, Zheng P, Wang X, et al.A preliminary evaluation of efficacy and safety of Wharton's jelly mesenchymal stem cell transplantation in patients with type 2 diabetes mellitus.Stem Cell Res Ther. 2014;5(2):57. |