中国组织工程研究 ›› 2014, Vol. 18 ›› Issue (51): 8254-8260.doi: 10.3969/j.issn.2095-4344.2014.51.011
• 口腔组织构建 oral tissue construction • 上一篇 下一篇
高糖介导人牙周膜细胞凋亡中bcl-2、bax的作用及机制
任伟伟1,陈书兰2,仇 静2,卢恕来2,刘海蓉2,刘世海3
- 1潍坊医学院口腔医学院,山东省潍坊市 261000;2青岛市市立医院口腔科,山东省青岛市 266071;3青岛大学医学院附属医院中心实验室,山东省青岛市 266555
-
出版日期:2014-12-10发布日期:2014-12-10 -
通讯作者:陈书兰,副主任医师,副教授,硕士生导师,青岛市市立医院口腔科,山东省青岛市 266071 -
作者简介:任伟伟,男,1978年生,湖北省十堰市人,汉族,潍坊医学院在读硕士,主要从事牙周病的基础及临床研究。 -
基金资助:青岛市科技局计划项目(2012-1-3-1- (15)-nsh)
Role and mechanism of bcl-2 and bax in high glucose-mediated apoptosis of human periodontal ligament cells
Ren Wei-wei1, Chen Shu-lan2, Qiu Jing2, Lu Shu-lai2, Liu Hai-rong2, Liu Shi-hai3
- 1 College of Stomatology, Weifang Medical University, Weifang 261000, Shandong Province, China
2 Department of Stomatology, Qingdao Municipal Hospital (Oral), Qingdao 266071, Shandong Province, China3 Central Laboratory, Affiliated Hospital of Qingdao University Medical School, Qingdao 266555, Shandong Province, China
-
Online:2014-12-10Published:2014-12-10 -
Contact:Chen Shu-lan, Associate chief physician, Associate professor, Master’s supervisor, Department of Stomatology, Qingdao Municipal Hospital (Oral), Qingdao 266071, Shandong Province, China -
About author:Ren Wei-wei, Studying for master’s degree, College of Stomatology, Weifang Medical University, Weifang 261000, Shandong Province, China -
Supported by:the Project of Science and Technology Bureau of Qingdao, No. 2012-1-3-1-(15)-nsh
摘要:
背景:高糖环境下人牙周膜细胞会发生凋亡,其中bcl-2,bax是否启动?如何发挥作用?此方面尚未见有文献报道。
目的:应用不同浓度葡萄糖影响人牙周膜细胞,观察细胞凋亡及bcl-2、bax基因表达的变化。
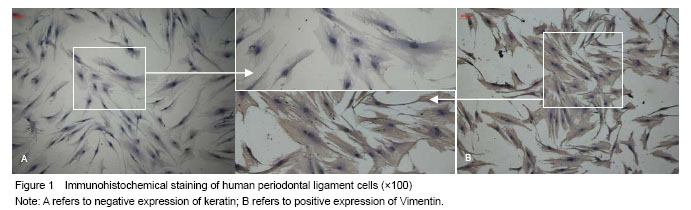
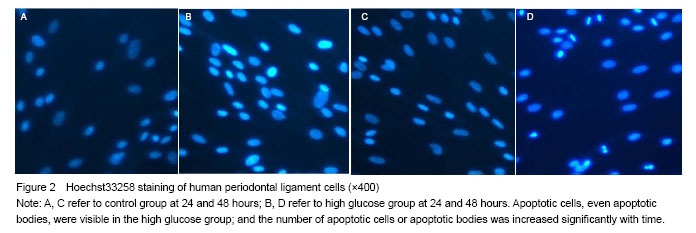
方法:原代培养并鉴定人牙周膜细胞,取5-8代细胞用于实验。采用5.5 mmol/L葡萄糖(生理对照组)和25 mmol/L葡萄糖(高糖组)作用细胞24 h和48 h;以Hoechst 33258免疫荧光染色观察人牙周膜细胞凋亡;以Real-time PCR检测bcl-2、bax表达。
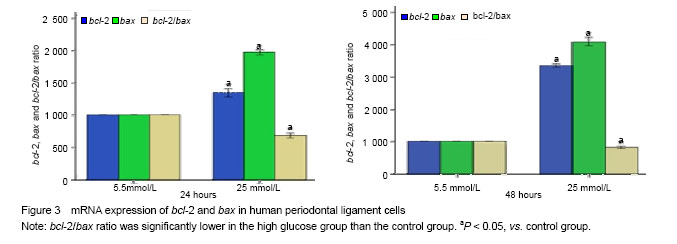
结果与结论:25 mmol/L葡萄糖促进人牙周膜细胞凋亡,bcl-2、bax表达显著高于对照组(P < 0.05);bcl-2/bax比值显著低于对照组(P < 0.05)。结果说明高糖可增加人牙周膜细胞凋亡,Bcl-2家族发挥了重要作用。
中图分类号:
引用本文
任伟伟,陈书兰,仇 静,卢恕来,刘海蓉,刘世海. 高糖介导人牙周膜细胞凋亡中bcl-2、bax的作用及机制[J]. 中国组织工程研究, 2014, 18(51): 8254-8260.
Ren Wei-wei, Chen Shu-lan, Qiu Jing, Lu Shu-lai, Liu Hai-rong, Liu Shi-hai. Role and mechanism of bcl-2 and bax in high glucose-mediated apoptosis of human periodontal ligament cells[J]. Chinese Journal of Tissue Engineering Research, 2014, 18(51): 8254-8260.
Results of Hoechst33258 staining
mRNA expression of bcl-2 and bax
| [1]Andriankaja OM, Sreenivasa S, Dunford R, et al. Association between metabolic syndrome and periodontal disease. Aust Dent J. 2010;55:252-259.
[2]Lui J, Wu Y, Ding Y, et al. Evaluation of serum levels of C-reactive protein and lipid profiles in patients with chronic periodontitis and/or coronary heart disease in an ethnic Han population. Quintessence Int. 2010;41:239-47.
[3]Armitage GC. Diagnosis of periodontal disease. J Periodontology. 2003;74:1237-1247.
[4]Orciani M, Trubiani O, Vignini A, et al. Nitric oxide production during the osteogenic differentiation of human periodontal ligament mesenchymal stem cells. Acta Histochemica. 2009;111(1):15-24.
[5]Bjelland S, Bray P, Gupta N, et al. Dentists, diabetes and periodontitis. Austr Dent J. 2002;47:202-207.
[6]Mustapha IZ, Debrey S, Oladubu M, et al. Markers of systemic bacterial exposure in periodontal disease and cardiovascular disease risk: a systematic review and meta-analysis. J Periodontol.2007;78(12):2289-2302.
[7]Sandberg GE, Sundberg HE, Fjellstrom CA, et al. Type 2 diabetes and oral health-a comparison between diabetic and non diabetic subjects. Diab Res and Clin Pract. 2000; 50(1): 27-34.
[8]Taylor GW, Borgnakke WS. Periodontal disease: associations with diabetes glycemic control and complications. Oral Dis. 2008;14(3):191-203.
[9]Lalla E. Periodontal infections and diabetes mellitus: when will the puzzle be complete? J Clin Periodont. 2007;34(11): 913-916.
[10]刘洪臣,布静秋.老年人常见的口腔疾病[J].中华老年口腔医学杂志,2008,6(4):I0018-I0020.
[11]Iwamoto Y, Nishimura F, Nakagawa M, et al. The effect of antimicrobial periodontal treatment on circulating tumor necrosis factor-alpha and glycated hemoglobin level in patients with type 2 diabetes. J Periodontol. 2001;72: 774-778.
[12]Grossi SG, Skrepcinski FB, DeCaro T, et al. Treatment of periodontal disease in diabetics reduces glycated hemoglobin. J Periodontol. 1997;68:713-719.
[13]Noble BS, Stevens H, Loveridge N, et al. Identification of apoptotic changes in osteocytes in normal and pathological human bone. Bone. 1997;20(3):273-282.
[14]Suzuki H, Wildhirt SM, Dudek RR, et al. Induction of apoptosis in myocardial infarction and its possible relationship to nitric oxide synthase in macrophages. Tissue Cell. 1996; 28(1):89- 97.
[15]Szabolcs M, Michler RE, Yang X, et al. Apoptosis of cardiac myocytes during cardiac allograft rejection. Relation to induction of nitric oxide synthase. Circulation. 1996;94(7): 1665-1673.
[16]蒋俊强,工忠朝,黎春晖,等.三种方法原代培养人牙周膜细胞[J]. 中国组织工程研究与临床康复,2010,14(23):4290-4294.
[17]Yuan YD, Miao S, Xie H. Effect of high glucose on the expression of transcription factor Scleraxis in periodontal ligament cells in vitro. Zhonghua Kou Qiang Yi Xue Za Zhi. 2008;43(11):668-670.
[18]Kim HS, Park JW, Yeo SI, et al. Effects of high glucose on cellular activity of periodontal ligament cells in vitro. Diabetes Res Clin Pract. 2006;74(1):41-47.
[19]Tan Z, Gong P, Zhao Q. Influence of basic fibroblast growth factors on the migration, proliferation of osteoblasts and periodontal ligament fibroblasts. Hua Xi Kou Qiang Yi Xue Za Zhi. 2005;23(3):201-203.
[20]Ohgi S, Johnson PW. Glucose modulates growth of gingival fibroblasts and periodontal ligament cells: correlation with expression of basic fibroblast growth factor. J Periodont Res. 1996;31:579-588.
[21]Chang PC, Chien LY, Chong LY, et al. Glycated matrix up-regulates inflammatory signaling similarly to Porphyromonas gingivalis lipopolysaccharide. J Periodontal Res. J Periodontal Res. 2013;48(2):184-193
[22]Kim SY, Lee JY, Park YD, et al. Hesperetin alleviates the inhibitory effects of high glucose on the osteoblastic differentiation of periodontal ligament stem cells. PLoS One. 2013;8(6):e67504.
[23]Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27: 519-550.
[24]Wong RK, Pettit AI, Quinn PA, et al. Advanced glycation end products stimulate an enhanced neutrophil respiratory burst mediated through the activation of cytosolic phospholipase A2 and generation of arachidonic acid. Circulation. 2003;108: 1858-1864.
[25]Allen EM, Mattews JB, O’Halloran DJ, et al. Oxidative and inflammatory status in type 2 diabetes patients with periodontitis. J Clin Peridontol. 2011;38:894-901.
[26]Preshaw PM, Alba AL, Herrera D, et al. Periodontitis and diabetes: a two way relationship. Diabetologia. 2012;55: 21-31.
[27]房明.高糖、糖基化终产物刺激下人牙周膜成纤维细胞生物学性能变化的体外研究[D].西安:第四军医大学,2013
[28]Katz J, Bhattacharyya I, Farkhondeh-Kish F, et al. Expression of the receptor of advanced glycation end products in gingival tissues of type 2 diabetes patients with chronic periodontal disease: a study utilizing immunohistochemistry and RT-PCR. J Clin Periodontol. 2005;32:40-44.
[29]D’Aiuto F, Parkar M, Andreou G, et al. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res. 2004;83:156-160.
[30]Navarro-Sanchez AB, Faria-Almeida R, Bascones-Martinez A. Effect of non-surgical periodontal therapy on clinical and immunological response and glycaemic control in type 2 diabetic patients with moderate periodontitis. J Clin Periodontol. 2007;34:835-843.
[31]Ganonal J, Bascones A, Acevedo A, et al. Apoptosis in chronic adult periodontitis analyzed by in situ DNA breaks, electron microscopy, and immunohistochemistry. J Periodontol. 2001;72(4):517-525.
[32]Liu J, Wu Y, Wang B, et al. High levels of glucose induced the Caspase-3/PARP signaling pathway, leading to apoptosis in human periodontal ligament fibroblasts. Cell Biochem Biophys. 2013;66(2):229-237.
[33]Schafer B, Quispe J, Choudhary V, et al. Mitochondrial outer membrane proteins assist Bid in Bax-mediated lipidic pore formation. Mol Biol Cell. 2009;20(8):2276-2285.
[34]Korsmeyer SJ. Bcl-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59(7 Suppl): 1693s-1700s.
[35]Kawarizadeh A, Bourauel C, Gotz W, et al. Early responses of periodontal ligament cells to mechanical stimulus in vivo. J Dent Res. 2005;84(10):902-906.
[36]Nowjack-Raymer RE, Sheiham A. Association of edentulism and diet and nutrition in US adults. J Dent Res. 2003;82: 123-126.
[37]Krall E, Hayes C, Garcia R. How dentition status and masticatory function affect nutrient intake. JADA. 1998;129: 1261-1269.
[38]Morais JA, Heydecke G, Pawliuk J, et al. The effects of mandibular two-implant overdentures on nutrition in elderly edentulous individuals. J Dent Res. 2003;82:53-58.
[39]Vernillo AT. Diabetes mellitus: relevance to dental treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91: 263-270.
[40]Vernillo AT. Dental considerations for the treatment of patients with diabetes mellitus. JADA. 2003;134:24s-33s. |
| [1] | 耿秋东, 葛海雅, 王和鸣, 李 楠. 基于网络药理学探讨龟鹿二仙胶治疗骨关节炎的作用及机制[J]. 中国组织工程研究, 2021, 25(8): 1229-1236. |
| [2] | 裴丽丽, 孙贵才, 王 弟. 丹酚酸B抑制骨髓间充质干细胞氧化损伤及促进分化为心肌样细胞[J]. 中国组织工程研究, 2021, 25(7): 1032-1036. |
| [3] | 李时斌, 赖 渝, 周 毅, 廖建钊, 章晓云, 张 璇. 激素性股骨头坏死发病机制及相关信号通路的靶点效应[J]. 中国组织工程研究, 2021, 25(6): 935-941. |
| [4] | 徐银琴, 史红美, 王光义. 通痹方热敷联合针刺治疗对退变椎间盘细胞凋亡相关基因Caspase-3、Bcl-2 mRNA的影响[J]. 中国组织工程研究, 2021, 25(5): 713-718. |
| [5] | 张雯雯, 金颂峰, 赵国梁, 宮丽鸿. 复方中药稳斑汤减少同型半胱氨酸诱导大鼠心肌微血管内皮细胞凋亡的作用机制[J]. 中国组织工程研究, 2021, 25(5): 723-728. |
| [6] | 刘 青, 万碧江. 针刀治疗胶原诱导性关节炎大鼠滑膜组织Bcl-2/Bax的表达[J]. 中国组织工程研究, 2021, 25(5): 729-734. |
| [7] | 谢崇新, 张 磊. 保留与不保留残端重建前交叉韧带术后膝关节退变的比较[J]. 中国组织工程研究, 2021, 25(5): 735-740. |
| [8] | 苏丽萍, 路子扬, 刘 丽, 张 巍, 苏天园, 胡夏韵, 蒲红伟, 韩登峰. 二乙酰吗啡致大鼠小脑颗粒神经元细胞凋亡过程中C-Jun、Cytc和Caspase-9的作用#br#[J]. 中国组织工程研究, 2021, 25(25): 3943-3948. |
| [9] | 左振魁, 韩佳瑞, 计树灵, 贺璐璐. 银杏酮酯预处理减轻辐射所致模型小鼠的急性肠损伤[J]. 中国组织工程研究, 2021, 25(23): 3666-3671. |
| [10] | 张 亮, 马晓燕, 王佳虹. 肾衰饮调控慢性肾功能衰竭大鼠肾脏细胞凋亡的机制[J]. 中国组织工程研究, 2021, 25(23): 3672-3677. |
| [11] | 谢 阳, 吕志宇, 张淑江, 龙 婷, 李作孝. 重组腺病毒介导神经生长因子转染实验性自身免疫性脑脊髓炎模型小鼠少突胶质细胞的凋亡及髓鞘化[J]. 中国组织工程研究, 2021, 25(23): 3678-3683. |
| [12] | 刘珂珂, 段 昕, 马向瑞, 张云涛. 以电纺丝膜为载体观察桂皮醛对高糖环境下成骨细胞的影响[J]. 中国组织工程研究, 2021, 25(22): 3500-3504. |
| [13] | 徐 彬, 杨秀书, 刘 旋, 王振兴. 尿液环境中肠上皮细胞及其凋亡因子半胱氨酸蛋白酶3、Bax和Bcl-2表达的变化[J]. 中国组织工程研究, 2021, 25(20): 3173-3177. |
| [14] | 宋世雷, 陈跃平, 章晓云, 李时斌 , 赖 渝, 周 毅. 五苓散治疗骨关节炎潜在分子机制及网络药理学与分子对接[J]. 中国组织工程研究, 2021, 25(20): 3185-3193. |
| [15] | 刘 昆, 谢 琳, 曹 军, 丁 宁, 徐灵博, 马胜超, 李桂忠, 姜怡邓, 卢冠军. 同型半胱氨酸致足细胞凋亡中FoxO1 DNA甲基化水平增高[J]. 中国组织工程研究, 2021, 25(2): 269-273. |
Design
Reagents and equipments for bcl-2 and bax in high glucose-mediated apoptosis of human periodontal ligament cells are as follows:
.jpg)
Two-step RT-PCR was adopted in the study. Hoechst33258 fluorescence staining was done as above. After that, 0.5 mL Trizol per well was added at 15-30 ℃ standing for 5 minutes, so nucleoprotein could be fully dissociated. After 0.1 mL chloroform was added, the plates were fully shaken vigorously for 15 seconds, standing at 15-30 ℃ for 2-3 minutes, followed by 12 000 r/min centrifugation at 4 ℃ for 15 minutes. Centrifuged samples were layered, and RNA was found in the upper aqueous phase. The supernatant was transferred to a new tube, added with 0.3 mL isopropanol, mixed by inversion and stood at 15-30 ℃ for 10 minutes. Then, some gelatinous precipitates appeared on the bottom of the tube, namely RNA. The RNA samples were centrifuged at 4 ℃ at 12 000 r/min for 10 minutes, and the supernatant was discarded. The retained precipitate was added with 0.5 mL of 75% ethanol containing 0.1% DEPC and mixed gently. After 12 000 r/min centrifugation for 5 minutes at 4 ℃, the supernatant was removed. The RNA samples were dried 10 minutes, and dissolved in 30 μL of DEPC at 55-60 ℃ for 10 minutes. Afterwards, the A260/A280 ratio was measured, Transcriptor First Strand cDNA Synthesis Kit was used for RNA reverse transcription and synthesis of cDNA. Primer design based on the coding region is shown in Table 1.
.jpg)
1 文章首次从凋亡形态学角度观察了高糖介导人牙周膜细胞凋亡影像,首次利用Real-time PCR检测了人牙周膜细胞高糖诱导下bcl-2,bax表达。 2 实验结果表明,Hoechst 33258免疫荧光操作简单,影像清晰,可直观辨识细胞凋亡情况。RT-PCR显示25 mmol/L葡萄糖促进人牙周膜细胞bcl-2、bax表达增强;bcl-2/bax比值下降。提示高糖可增加人牙周膜细胞凋亡,Bcl-2家族发挥了重要作用。
基金资助: 青岛市科技局计划项目(2012-1-3-1- (15)-nsh)
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||