Design
Case-control study.
Time and setting
All patients with acute myocardial infarction complicated by heart failure were admitted in the Department of Cardiology, Jinqiu Hospital of Liaoning Province, China from August 2005 to September 2006.
Subjects
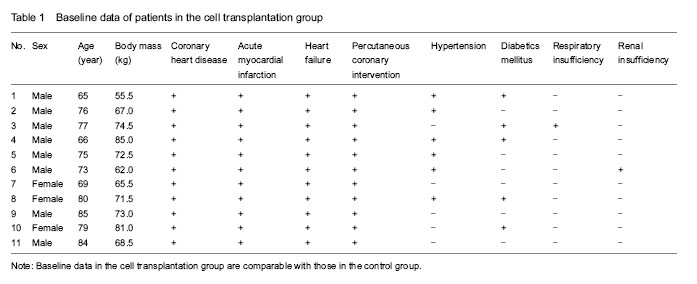
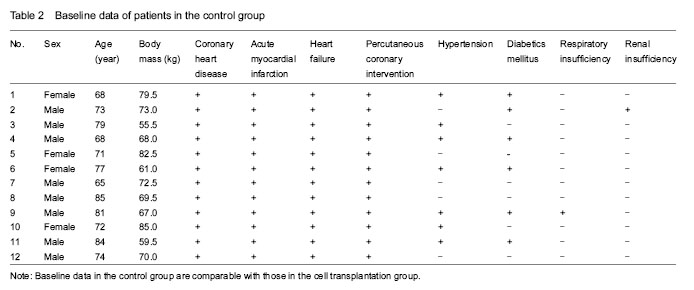
Twenty-three patients with acute ST-segment elevation myocardial infarction complicated by heart failure, who had undergone emergency coronary angiography and percutaneous coronary intervention with drug-eluting stent implantation, were enrolled, including 16 males and 7 females, aged 65-85 years with a mean age of (75±10) years, weighing 55.5-85.0 kg with a mean of (70±15) kg. Of the 23 patients, 13 cases had hypertension, 11 cases had diabetes, 2 cases had respiratory insufficiency, and 2 cases had renal insufficiency.
Diagnostic criteria: (1)persistent chest pain ≥ 30 minutes; (2)electrocardiogram showed 2 mm ST segment elevation in at least two adjacent or contiguous leads; (3)elevation of serum creatine kinase MB isoenzyme or troponin I; (4)presence of dyspnea, coughing, cyanosis, irritability and other symptoms of heart failure.
Inclusion criteria: (1)acute myocardial infarction complicated by heart failure; (2)cardiac function at admission ≥ grade II of Killip’s classification; (3)conventional drug therapy is invalid; (4)the left ventricular ejection fraction < 45%; (5)patients and their families signed an informed consent form, and the therapeutic schedule was approved by the ethics committee of the hospital.
Exclusion criteria: (1)patients who could not receive or disagreed with percutaneous coronary intervention; (2)presence of angina and cardiogenic shock prior to transplantation; (3)with history of cancer or other fatal diseases that can impact patient’s short-term survival; (4)anemia and coagulation disorders; (5)progressive hepatic failure.
According to patient’s willness, the 23 patients were divided into: cell transplantation group (n=11), including 8 males and 3 females, aged 65-85 years; and control group (n=12), including 8 males and 4 females, aged 65-85 years. Baseline characteristics of the two groups were similar and comparable.
Methods
In the control group, patients were routinely given anti-platelet aggregation, crown expansion, diuretic, cardiac, and other drugs to improve the ventricular remodeling at admission, and then, received the emergency coronary angiography and percutaneous coronary intervention with drug-eluting stent implantation.
In the cell transplantation group, stem cell transplantation was conducted 5 days after percutaneous coronary intervention.
Mobilization and collection of peripheral blood stem cells: granulocyte colony-stimulating factor at a dose of 300-
600 μg/d was injected subcutaneously for 5 consecutive days. At day 6, peripheral blood samples were collected via cubital vein puncture. Collected blood samples were placed via a closed sterile pick device into a blood cell separator for extraction of peripheral blood stem cells. Hematopoietic stem cells were collected and stored in blood bags, and the remaining blood flowed back to the patient’s body. Finally, the total amount collected was 50-100 mL. The isolated stem cells were counted by flow cytometry to collect CD34+ cells (about 1×108).
Cell transplantation: At the day of transplantation, the femoral artery access was established under the sterile environment in the conduit room. Ischemic coronary angiography was performed to introduce a microcatheter into the guiding catheter. Then, the suspension of peripheral blood stem cells was injected via the microcatheter into the distal end of infarcted vessels, 2.0-3.0 mL every 3 minutes, totally 30 mL (about 1×108 CD34+ cells). After injection, balloon occlusion was done for 3 minutes. We observed whether intraoperative arrhythmia, micro-embolism and hypotension occurred. If patients presented with chest pain, chest tightness, unbearable, or severe arrhythmia, the balloon occlusion time could be reduced appropriately.
Main outcome measures
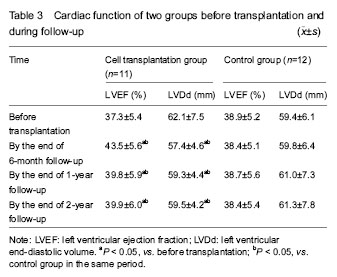
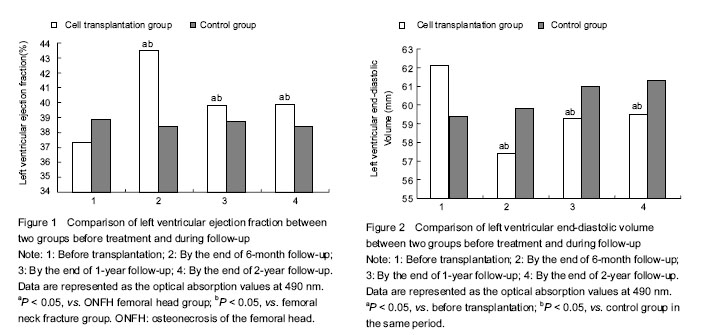
(1)Cardiac function prior to, 6 months, 1 year, 2 years after treatment in the two groups was observed; Echocardiography (SONO7500, Philips) was used by a designated physician from the ultrasound department to evaluate the left ventricular ejection fraction and left ventricular end-diastolic volume before and after treatment. (2)Safety monitoring: we observed whether the fever, cancer and other adverse reactions occurred, and whether there were new myocardial ischemic damage, inflammation and malignant arrhythmias reactions.
Statistical analysis
Measurement data were expressed as mean±SD and statistically analyzed by SPSS 10.0 statistical software. Student’s t test was performed between two groups, and a value of P < 0.05 was considered statistically different.