中国组织工程研究 ›› 2024, Vol. 28 ›› Issue (29): 4717-4725.doi: 10.12307/2024.540
• 生物材料综述 biomaterial review • 上一篇 下一篇
基于不同组织和器官角度回顾PGC-1α在运动抗衰老中的作用
李兆进1,郑鹏程2,孔健达2,朱腾旗1,姜付高1
- 曲阜师范大学,1体育学部,2体育科学学院,山东省济宁市 272000
-
收稿日期:2023-10-20接受日期:2023-11-20出版日期:2024-10-18发布日期:2024-03-23 -
通讯作者:姜付高,博士,教授,博士生导师,曲阜师范大学体育学部,山东省济宁市 272000 -
作者简介:李兆进,男,1977年生,山东省青岛市人,汉族,曲阜师范大学体育科学学院在读博士,副教授,硕士生导师,主要从事体育教学和体育管理研究和工作。 -
基金资助:山东省社科规划一般项目(21CTYJ08),项目负责人:朱腾旗
Review of PGC-1α role in exercise anti-aging in different tissues and organs
Li Zhaojin1, Zheng Pengcheng2, Kong Jianda2, Zhu Tengqi1, Jiang Fugao1
- 1School of Physical Education, 2College of Sports Science, Qufu Normal University, Jining 272000, Shandong Province, China
-
Received:2023-10-20Accepted:2023-11-20Online:2024-10-18Published:2024-03-23 -
Contact:Jiang Fugao, PhD, Professor, PhD supervisor, School of Physical Education, Qufu Normal University, Jining 272000, Shandong Province, China -
About author:Li Zhaojin, PhD candidate, Associate professor, Master’s supervisor, School of Physical Education, Qufu Normal University, Jining 272000, Shandong Province, China -
Supported by:General Project of Shandong Provincial Social Science Planning, No. 21CTYJ08 (to ZTQ)
摘要:
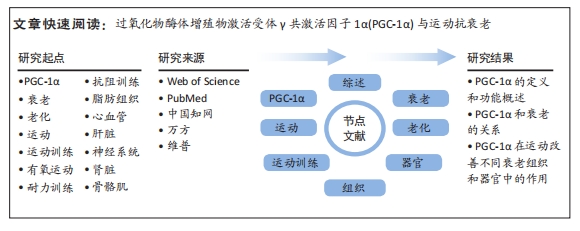
文题释义:
PGC-1α:中文全称为过氧化物酶体增殖物激活受体γ共激活因子1α,是一种转录共激活因子,主要在肝脏、肌肉和脑等组织中表达,能够调节细胞能量代谢和线粒体功能,并参与调节血糖代谢、脂肪酸氧化和肌肉适应性等生物学过程,其功能受多种生理和环境因素的调控,包括运动、饥饿、冷暴露和泌乳酸。衰老:是人类或动物体内发生的一系列生理过程,表现为身体功能逐渐下降、肌肉减少、皮肤变得松弛、免疫系统减弱、记忆力下降等。随着年龄的增长,人体的各种系统逐渐失去原有的功能和弹性,导致身体功能减弱,容易受到疾病的侵袭。衰老是不可逆转的过程,但通过健康的生活方式和医学干预可以延缓衰老的进程。
背景:过氧化物酶体增殖物激活受体γ共激活因子1α(peroxisome proliferators-activated receptors gamma co-activator 1α,PGC-1α)和衰老密切相关,且其在运动抗衰老中发挥着重要的调控作用,但缺乏从不同组织和器官视角下PGC-1α在运动抗衰老中作用的相关综述。
目的:详细回顾PGC-1α在运动抗衰老中的作用,并从不同组织和器官的角度探讨其调控情况。方法:于2023-05-01/07-01进行文献检索。检索范围包括自各数据库建库至2023年7月,并在Web of Science、PubMed、中国知网、万方和维普数据库上进行检索。中文检索词:“PGC-1α,过氧化物酶体增殖物激活受体γ共激活因子1α,PPARGC1A,衰老,运动,老年人等”;英文检索词:“PGC-1α,aging,exercise,exercise training,older adults”。运用布尔逻辑运算符将检索词连接进行检索,并制定了相应的检索策略。根据纳入和排除标准进行筛选,最终纳入文献83篇进行综述分析。
结果与结论:①PGC-1α是一个重要的转录共激活因子,在维持线粒体功能、调控能量代谢和适应不同代谢需求方面发挥着关键的调节作用。②在线粒体衰老中的多种功能,在多种细胞类型中的调节作用,在多种细胞类型中发挥着重要的调节作用,与炎症途径和氧化还原控制的关系及其相关蛋白修饰和表观遗传变化。③PGC-1α的表达水平能够被运动训练提高,并通过调节线粒体生物发生、能量代谢和抗氧化应激等途径发挥积极的作用,其在运动改善脂肪组织衰老、心血管老化、神经系统老化、肾脏衰老、骨骼肌衰老和肝脏老化等中发挥重要作用。④课题组专家建议未来研究方向包括探索不同类型、强度和时长的运动对PGC-1α表达的调节影响,研究PGC-1α的蛋白修饰和表观遗传变化的调节机制,以及加强对PGC-1α在不同衰老相关疾病中的作用机制的研究。
https://orcid.org/0009-0000-0977-801X(李兆进);https://orcid.org/0000-0002-7674-2372(姜付高)
中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
中图分类号:
引用本文
李兆进, 郑鹏程, 孔健达, 朱腾旗, 姜付高. 基于不同组织和器官角度回顾PGC-1α在运动抗衰老中的作用[J]. 中国组织工程研究, 2024, 28(29): 4717-4725.
Li Zhaojin, Zheng Pengcheng, Kong Jianda, Zhu Tengqi, Jiang Fugao. Review of PGC-1α role in exercise anti-aging in different tissues and organs[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(29): 4717-4725.
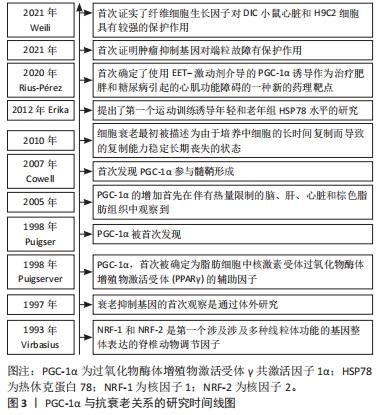
综上,PGC-1α是一个重要的转录共激活因子,在维持线粒体功能、调控能量代谢和适应不同代谢需求方面发挥着关键的调节作用。通过活化PGC-1α,能够促进细胞的线粒体健康,提高氧化代谢和抗氧化能力,从而对运动抗衰老产生积极影响。然而,还有一些关于PGC-1α的研究内容尚不清楚,如其具体的调控机制以及在其他生理和病理过程中的作用等,这些问题需要进一步研究来解答。PGC-1α与抗衰老关系的研究时间线图见图3。
2.2 PGC-1α和衰老的关系 PGC-1α与衰老存在着密切的关系,并在衰老过程中具有多种功能。首先,PGC-1α通过调节线粒体生物合成和重塑、脂肪酸的运输和氧化作用、炎症反应、抗氧化代谢以及葡萄糖代谢的调节,参与调节线粒体衰老中的多方面功能,其功能受到多种刺激调节,如β肾上腺素敏感性减少、端粒酶反转录酶缺陷等。其次,在多种细胞类型中,如肌肉、神经、脂肪、心脏和胰岛细胞等中发挥调节作用,但在衰老过程中受到多种信号通路的负调控,与脂肪酸氧化受损、胰岛素抵抗等相关。此外,PGC-1α在能量代谢调控和氧化还原状态平衡中扮演着重要的角色,但在衰老过程中会受到炎症反应和胰岛素信号通路的影响。最后,蛋白修饰和表观遗传变化在调节PGC-1α功能中也起着关键作用,如乙酰化、磷酸化、DNA甲基化和超甲基化等调控机制。图4显示了PGC-1α和衰老的关系。
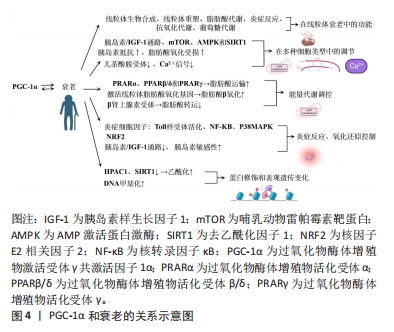
2.2.1 PGC-1α在线粒体衰老中的多种功能 PGC-1α是一种具有多种功能的蛋白,在衰老中发挥着调节作用。研究表明,PGC-1α能够调节线粒体的生物合成和重塑、脂肪酸的运输和氧化作用、炎症反应、抗氧化代谢以及葡萄糖代谢的调节[9]。尽管尚不清楚衰老引起PGC-1α减少的具体原因,但β肾上腺素敏感性减少可能会抑制PGC-1α的表达[12]。另外,研究发现,端粒酶反转录酶缺陷导致PGC-1α的下调,从而引起线粒体受损,这表明端粒酶功能障碍与细胞器受损在衰老过程中存在关联[13]。
有些研究还发现,细胞核中线粒体DNA的突变、慢性组织不使用以及与衰老相关的疾病可能直接或间接导致PGC-1α减少[14-15]。这些发现进一步表明PGC-1α的下降可能是衰老进程中的一个重要因素。另外,PGC-1α调节的所有功能都相互关联,并依赖于个体线粒体、整个细胞以及细胞器网络的完整性[1]。因此,维持PGC-1α水平对于细胞和整个机体的正常功能至关重要。在一项对转基因小鼠的研究中发现,PGC-1α水平较高的小鼠寿命更长,这可能与其能量代谢途径的增加、肌肉完整性以及再生相关的mRNA水平增加有关[16]。
综上所述,PGC-1α在衰老中发挥着多种功能,并受到多种刺激的调节。尽管尚未完全了解其调节机制和在衰老过程中的具体作用,但通过进一步的研究和探索,能够更好地理解PGC-1α的功能,并找到调节其水平的途径,从而对抗衰老过程。
2.2.2 PGC-1α在多种细胞类型中的调节作用 PGC-1α在许多细胞类型中均参与代谢,在肌、神经、脂肪心脏和胰岛细胞等多种细胞中发挥调节作用。然而,在衰老过程中PGC-1α的功能受到负调控,导致其代谢调节能力明显下降,这种负调控涉及多个信号通路,如胰岛素/类胰岛素生长因子1[17]、哺乳动物雷帕激酶[18]、AMP激活的激酶以及抑制剂sirtuin 1的活性[19-20],这些信号通路的活性降低与与衰老相关的胰岛素抵抗和脂肪酸氧化受损密切相关。在细胞水平上,PGC-1α与许多转录因子相互作用,包括核呼吸因子和线粒体转录因子A等[21]。然而,在衰老过程中,PGC-1α对这些转录因子的激活能力出现明显下降[22]。进一步的研究发现,衰老导致肝脏、大脑、心脏和骨骼肌中的β肾上腺素受体逐渐减少,从而导致对儿茶酚胺的反应性降低,可能是衰老过程中PGC-1α水平降低的原因之一[22]。另外,衰老还会降低细胞内Ca2+信号系统的功能,包括大鼠心肌和果蝇胸肌中的信号传导,这种降低可能影响到PGC-1α的激活,并对其功能产生负面影响[23]。
综上所述,PGC-1α在多种细胞类型中发挥着重要的调节作用。但在衰老过程中,其功能受到多种信号通路的负调控,这种调控与脂肪酸氧化受损、胰岛素抵抗的发生密切相关,并且与转录因子的激活能力下降、儿茶酚胺受体的减少以及细胞内Ca2+信号系统的降低有关。进一步研究PGC-1α的功能与调节机制对于理解衰老相关疾病的发生和发展具有重要意义。然而,目前对于PGC-1α在不同细胞类型及衰老过程中的具体调控机制仍需进一步研究。
2.2.3 PGC-1α和能量代谢调控的关系 PGC-1α在能量代谢调控中起着重要作用,其与过氧化物体增殖物激活受体相连,主要包括过氧化物酶体增殖物激活受体γ共激活物1α、过氧化物酶体增殖物激活受体γ共激活物1β/δ和过氧化物酶体增殖物激活受体γ等,这调节脂肪酸的运输和利用[12]。具体来说,PGC-1α通过激活线粒体脂肪酸氧化基因,促进脂肪酸的β-氧化转化为完整的β-氧化[24]。大量表达PGC-1α会导致脂肪酸的β-氧化转化为完整的β-氧化,这被视为代谢重编程的一种形式[25]。另外,p38线粒体激活蛋白激酶的激活使得PGC-1α的激活对于刺激肝葡萄糖新生来说是必不可少的[9],这意味着PGC-1α的活化状态对于维持肝葡萄糖新生的正常功能至关重要。β肾上腺素受体的激活与衰老有关,会导致脂肪储存的转运减少,从而降低PGC-1α的表达水平[12],这表明PGC-1α可能在调控脂肪代谢中发挥着重要的作用。另外,PGC-1α的表达水平下调与一些不良代谢效应密切相关,如体内脂肪质量增加、胰岛素抵抗和炎症等[26]。然而,PGC-1α的丧失并不会影响褐色脂肪细胞的分化,但会减少关键的发热基因的诱导效应[26],这一发现揭示了PGC-1α在能量代谢调控中的特定作用。
2.2.4 PGC-1α与炎症途径和氧化还原控制的关系 当前已有研究证据已经揭示了PGC-1α在衰老过程中的重要作用,其通过连接氧化还原控制和炎症途径调控着细胞的能量代谢和氧化还原状态[27]。PGC-1α水平的降低会导致炎症反应逐渐增加,主要是因为激活了核因子κB和调控p38线粒体激活蛋白激酶的影响,从而导致炎症细胞因子过度表达[28]。同时,激活的炎症细胞因子和Toll样受体还会降低PGC-1α在孤立巨噬细胞中的表达水平[29],另外,PGC-1α的功能与抗氧化剂转录因子红细胞生成相关因子2的调节密切相关,这一调控在大脑和肌肉中平衡能量供应和氧化还原状态[30]。然而,PGC-1α的功能可能会受到干扰,从而影响其调控能量代谢和氧化还原状态的能力[30]。
另外,PGC-1α与胰岛素信号通路亦密切相关。当PGC-1α水平减少时,会抑制胰岛素/胰岛素样生长因子1信号通路,进而影响胰岛素敏感性[31]。研究发现,在衰老过程中,PGC-1α的表达水平增加与胰岛素分泌和胰岛功能的改善相关[32]。这一机制可能通过调节胰岛素受体底物1/胰岛素受体底物2的比例来实现,这在精确调控胰岛素信号中具有重要作用[33]。另外,肌肉中缺乏PGC-1α亦会引发系统性炎症反应和胰岛素抵抗。PGC-1α的减少导致转化生长因子β信号通路和AMP激活蛋白激酶的活性降低,从而影响肌肉对葡萄糖的摄取能力,甚至可能对全身的葡萄糖稳态产生影响[34]。
综上所述,PGC-1α在调控能量代谢和氧化还原状态平衡中具有重要的作用,其水平下降会导致炎症反应增加和胰岛素信号通路异常,从而影响细胞能量代谢和整体代谢状态。然而,需要进一步的研究来揭示其作用的详细机制。
2.2.5 PGC-1α的蛋白修饰和表观遗传变化 在PGC-1α的转位和激活过程中,蛋白质翻译后的修饰起着关键作用。研究表明,乙酰化和磷酸化等上游信号能够调节PGC-1α的转录和翻译过程[28]。另外,针对衰老骨骼肌的研究揭示了AMP激活蛋白激酶磷酸化和p38线粒体激活蛋白激酶的减少现象,同时磷酸化CREB与CREB的比例显著降低,从而降低了DNA结合[35]。具体而言,衰老过程中,乙酰化修饰明显增加,这会对PGC-1α的功能产生负面影响,尤其是对线粒体功能和代谢的影响[36]。研究表明,这种现象可能与老年大脑内皮细胞中组蛋白去乙酰化酶1的减少有关,组蛋白去乙酰化酶的消耗降低了去乙酰化因子1水平,进而促进了PGC-1α的乙酰化过程[36]。另外,乙酰化过程还会降低PGC-1α的转录活性,从而影响线粒体下游基因的表达[36]。
另外,衰老还降低了去乙酰化因子1的去乙酰化作用,导致肝脏中PGC-1α蛋白的mRNA水平降低[37]。与此同时,衰老过程与DNA甲基化等表观遗传变化密切相关。研究发现,老年帕金森病患者的脑中PGC-1α基因启动子近端存在非经典胞嘧啶甲基化现象,并伴随mRNA水平的下降[38],这种现象与内质网应激和炎症信号的相互作用有关。另外,证据还表明,PGC-1α的超甲基化与2型糖尿病老年患者线粒体水平的下降同时发生。衰老过程可能导致核小体在PGC-1α的结合位点上发生重构[39]。
综上所述,蛋白质修饰和表观遗传变化在调节PGC-1α功能中起着关键作用。进一步研究这些调控机制能够揭示衰老过程中PGC-1α功能丧失的原因,并有助于开发针对衰老相关疾病的治疗策略。但是,对于这些调控机制的具体意义仍不清楚,还需要进一步的研究来解答。
2.3 PGC-1α在运动改善不同衰老组织和器官中的作用 运动被广泛认为是一种有效的干预措施,能够延缓衰老过程并改善不同组织和器官的功能。在过去的研究中,PGC-1α被发现在运动中起到了重要的调控作用。具体而言,PGC-1α的表达水平能够被运动训练提高,并通过调节线粒体生物发生、能量代谢和抗氧化应激等途径发挥积极的影响。以下内容详细回顾了PGC-1α在运动改善不同衰老组织和器官中的作用,主要包括PGC-1α在运动改善脂肪组织衰老、心血管老化、神经系统老化、肾脏衰老、骨骼肌衰老和肝脏老化等中的作用。具体来说,在脂肪组织中,运动可增加P GC-1α含量、提升线粒体生物合成和呼吸活性,从而减轻体脂和改善老化相关的体脂肪;在心血管系统中,运动通过调节PGC-1α来预防心脏结构和功能下降,提高蛋白激酶α亚单位1蛋白含量,改善心脏状况,并增加血管生成;在神经系统中,运动调控PGC-1α促进树突棘和突触的形成,改善神经发生,提高与衰老相关的神经发生和线粒体功能,进而提供神经保护作用;在肾脏中,运动能提高肾脏中PGC-1α的表达,进而调节肾脏纤维化、肾脏功能和肾脏线粒体功能,改善肾脏功能;在骨骼肌中,适当的运动可调节肌肉中PGC-1α的表达,并增强其对线粒体健康的作用,改善衰老导致的肌肉退化和线粒体功能恶化;在肝脏中,PGC-1α参与调节内质网应激、脂肪酸氧化等多个生理功能改善肝脏老化和功能。图5显示了PGC-1α在运动改善不同衰老组织和器官中的作用。
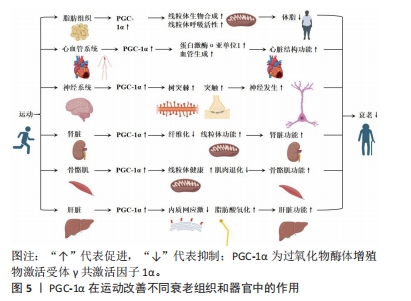
2.3.1 PGC-1α在运动改善脂肪组织衰老中的作用 在脂肪组织中,衰老会引起诸多变化,其中之一便是线粒体功能受损[40]。这一损伤对全身健康产生负面影响,与代谢和脂肪细胞分化紧密相连,因为线粒体掌控着氧化磷酸化、脂肪酸代谢和氧化还原[41]。灰褐色脂肪细胞高度表达PGC-1α,负责增强与热原性相关的基因表达[2]。
运动对改善脂肪组织衰老具有积极作用,尤其是在上调白色脂肪组织中PGC-1α的mRNA水平方面。ZIEGLER等[42]发现,抗阻训练和耐力训练可以减少附睾脂肪质量和脂肪细胞大小,并伴有抗炎表型增强。THIRUPATHI等[43]的研究表明,运动训练能够通过调控脂肪组织上线粒体的活性来降低与衰老相关的体脂肪。这说明,无论是抗阻性训练还是有氧运动,都能增加棕色脂肪组织中线粒体调节蛋白的含量和呼吸链活性,同时降低老龄大鼠的体脂肪和脂肪组织。另外,SUN等[44]的研究发现,终身跑步机运动能够提升肾旁脂肪组织中PGC-1α,UCP1和COX Ⅳ蛋白的水平,有效增强老龄大鼠的线粒体生物合成和呼吸活性。这不仅证实了不同类型的运动可以部分恢复衰老导致的脂肪组织线粒体功能下降,还揭示了定期运动的重要性。此外,研究表明低温下运动可以更有效地诱导大鼠腹股沟AMPK,PGC-1α和UCP1蛋白过表达,促进白色脂肪棕色化,从而降低体脂、减轻体质量[45]。另外,付鹏宇等[46]的研究显示低氧运动可以通过增加UCP1和PGC-1α mRNA的相对含量、增强PGC-1α蛋白的表达以及提高棕色脂肪的活性来促进白色脂肪的棕色化。这说明,运动对脂肪组织中PGC-1α的影响在多个方面都是积极的。然而,后期研究需要进一步探讨不同类型运动对脂肪组织的影响机制及其在防治衰老相关疾病中的作用。
综合来看,PGC-1α在运动改善脂肪组织老化过程中扮演着关键角色。运动通过调节脂肪组织上线粒体的活性和增强PGC-1α的表达,从而改善线粒体功能、减轻脂肪组织质量、增强抗炎表型和脂肪分解。这些研究结果为深入探讨脂肪组织老化机制提供了重要参考。然而,脂肪组织老化的具体内容仍存在许多不明之处,有待进一步研究。PGC-1α在运动改善脂肪组织衰老中作用的相关研究进展见表1。
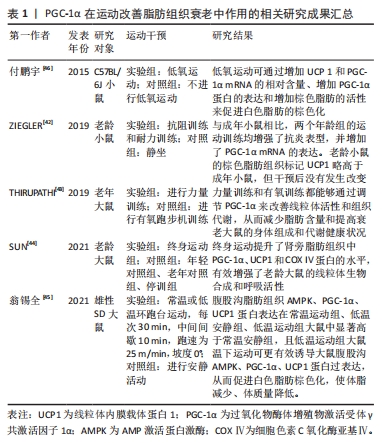
2.3.2 PGC-1α在运动改善心血管老化中的作用 心血管老化导致心脏结构和功能下降,并增加心血管疾病的发病率和死亡率[3]。适应性心脏重塑是导致这些改变的结果,其中包括心肌线粒体生成和代谢适应的关键调节因子PGC-1α的下降[3]。PGC-1α下降会影响能量代谢、兴奋-收缩过程和钙信号稳态。尽管PGC-1α的急剧减少不能引发衰老表型,但增加这种分子可能会减少老年小鼠心脏的病理性重塑[21]。
运动和PGC-1α在改善心血管老化中具有重要作用。BAYOD等[47]的研究发现,适度运动能够通过激活果蝇心脏中的PGC-1α来改善运动能力和寿命。另外,CHEN等[48]进行了一项研究发现,运动训练能增加左心室中去乙酰化因子1、PGC-1α和 AMP激活的蛋白激酶α亚单位1蛋白的含量,并抑制与衰老相关的炎症信号通路,具体而言,游泳能够改善老年大鼠心脏状况,减少心脏细胞排列的混乱,且长期运动训练能通过提高PGC-1α表达和激活去乙酰化因子1的功能来保护老年心脏,增加血管内皮生长因子水平和过氧化氢酶活性,从而预防与年龄相关的疾病。
YEO等[49]的研究评估了老年大鼠左心室对不同类型运动对PGC-1α的调节作用,发现有氧运动能够增加老年心肌组织中PGC-1α的含量,并刺激PGC-1α调控的血管内皮生长因子途径,促进血管生成。有氧运动还能够保护老年大鼠主动脉线粒体完整性,减轻肿胀和DNA含量,并恢复复合物Ⅰ/Ⅲ的活性和电子耦合能力[50]。另外,有氧运动训练还能够改善老年主动脉的弹性和功能,通过降低胶原蛋白含量和增加弹性蛋白含量,改善脉搏波速度和主动脉硬化,并通过增加内皮介导的血管舒张来改善内皮功能[51]。同时,自愿有氧运动亦能够增强受损动脉的恢复能力,调控老年小鼠动脉健康的关键因子,维持动脉的线粒体健康,并且血流限制与中等强度耐力运动相结合,能够改善心脏的收缩能力,通过增加PGC1-α和Klotho的表达来减轻左室舒张压[52]。另外,短期运动干预使得心脏中PGC-1α的表达上调,同时降低了心脏和全身的炎症反应。然而,这种运动干预还导致PGC-1α下游的转录因子如核重复序列因子1和一些呼吸链基因的表达降低[53]。
综上所述,运动对心脏和动脉有广泛的生理效应,促进了适应性改变,维持体内稳态,预防与年龄相关的疾病。但运动类型、强度和时间对适应效果有差异,需要根据具体情况进行选择。这些研究为进一步研究PGC-1α在运动改善神经系统老化中的作用提供了一定的理论基础,但仍有一些问题有待科学家进一步探索和解答。PGC-1α在运动改善心血管老化中作用的相关研究进展见表2。
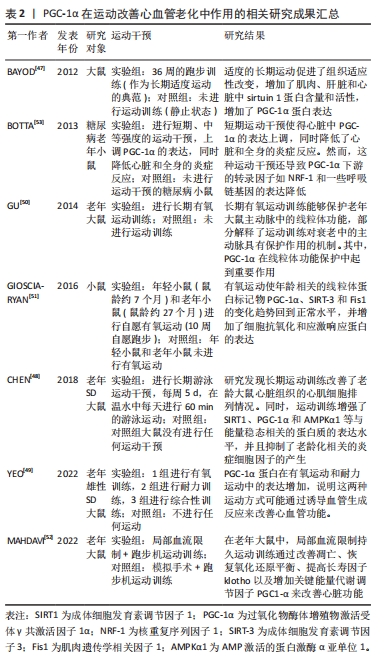
2.3.3 PGC-1α在运动改善神经系统老化中的作用 PGC-1α在运动改善神经系统老化中发挥重要作用。神经需要大量正常线粒体来满足高能量需求[54],因此线粒体的质量和活动对神经功能至关重要。线粒体的下降与衰老、神经退行性疾病和痴呆症有关,影响神经功能[54]。尽管突触和认知功能障碍是多个因素共同作用的结果,但线粒体在这些过程中起到重要作用,故适当维持细胞器功能能够对抗与衰老相关的变化[55]。
研究显示,PGC-1α能够促进树突棘和突触的形成,改善突触发生和神经发生[4]。高于乳酸阈值的跑步机运动能够影响与生物能量相关的靶点[56]。有研究发现,老年小鼠在运动后,大脑中PGC-1α、雷帕霉素靶蛋白水平、磷酸化的雷帕霉素靶蛋白水平以及线粒体DNA复制数都有所增加[56],这说明运动能够改善与衰老过程有关的神经发生和线粒体功能障碍。高强度训练能够激活部分脑线粒体生物发生,有利于维持组织健康。游泳运动训练能够增强海马细胞密度,并上调老化大鼠海马中的胰岛素样生长因子1R/磷脂酰肌醇三酸激酶/蛋白激酶B轴和AMP激活的蛋白激酶/去乙酰化酶/PGC-1α通路[55]。运动逆转了衰老对凋亡途径和炎症途径的不利影响,对细胞存活和细胞寿命的维持至关重要[55]。另一项研究发现,经过运动的老年小鼠中耦合复合物Ⅰ到Ⅲ的酶活性和与动力蛋白1相关的表达增强,但脑部线粒体蛋白含量和线粒体生物发生标志物则未见变化[57],研究表明脑部线粒体功能可能受到其他途径的影响。因此,运动通过多种机制激活复杂的结果,而持续的运动模拟对神经保护的反应很可能需要涉及多个分子途径。另外,KARLSSON等[58]评估了肌肉中PGC-1α过表达对神经发生的影响,并研究了持久性运动是否能进一步增强这些影响,但其并未观察到其他肌肉PGC-1α过表达或运动诱导的改善衰老相关神经退化的其他积极效应[58],其中一个原因可能是无法准确测量自愿性运动的强度和量;另外,由于大脑对葡萄糖代谢的需求更大,脑部线粒体对运动的影响可能与其他器官有所不同[59]。因此,需要进一步研究来确定对运动引发神经系统变化的重要因素。
综上所述,通过正常功能的线粒体和适当的运动能够改善神经系统老化,并提供神经保护作用。了解和研究运动对神经系统变化的机制,将有助于开发更有效的干预措施来延缓神经系统老化和相关疾病的发展。PGC-1α在运动改善神经系统老化中作用的相关研究进展见表3。
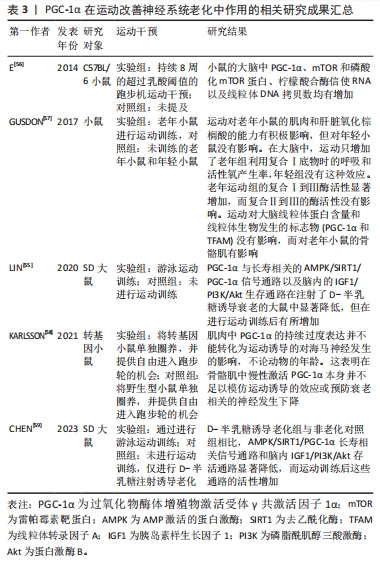
2.3.4 PGC-1α在运动改善肾脏衰老中的作用 PGC-1α是一个在维持肾脏健康方面具有重要作用的蛋白。研究发现,PGC-1α可能起到保护肾脏免受损伤的关键作用[5]。具体来说,突变的PPARGC1A基因能够提高PGC-1α蛋白的水平和氧化代谢,从而保护肾脏免受肾病伤害[5],这个发现为预防和治疗肾脏疾病提供了新的可能性。一项研究还发现,在老年小鼠中,PGC-1α失活会导致尿钠排泄增加和由代谢应激诱导的肾脂肪变性加重[60],研究表明PGC-1α在衰老过程中对肾脏保护作用的减弱。然而,亦有研究揭示了激活PGC-1α有助于改善肾脏功能。例如,激活PGC-1α能够在缺血后显著提高局部NAD前体烟酰胺的水平、减少脂肪积累[61],这为通过激活PGC-1α来改善肾脏损伤和促进肾脏再生提供了潜在的治疗策略。另外,PGC-1α还通过转录因子EB介导的自噬过程,改善了顺铂诱导的肾脏损伤中的线粒体功能障碍,并减轻了肾脏纤维化的发生[62-63]。因此,通过调节PGC-1α和TFEB的活性,能够有效防止肾脏纤维化和保护肾脏功能。
运动被认为是改善肾功能并预防糖尿病并发症的有效方式。适度的有氧运动方案能够增加肾脏中PGC-1α蛋白的含量,并提高柠檬酸合酶和线粒体复合物的活性[64],这进一步支持了PGC-1α在肾脏健康方面的重要作用。另外,运动训练还能够避免去乙酰化酶的下调,可能通过降低肾脏核转录因子κB乙酰化来达到效果[65],这一发现为解释为什么运动对糖尿病引起的肾损伤有益,并提供了一种潜在机制。另外,一项研究发现,在慢性肾脏疾病小鼠中,自噬介导的蛋白质降解导致线粒体功能障碍和三磷酸腺苷产生减少[66],这对于了解慢性肾脏疾病中的肌肉消耗机制,并开发相应的干预措施具有重要临床意义。PGC-1α的研究结果提示了其在慢性肾脏疾病中的作用,为进一步研究和治疗该疾病提供了新的靶点。总之,PGC-1α在运动改善肾脏衰老中发挥了重要作用,但仍有一些问题尚待解决。
综上所述,通过调节PGC-1α信号通路和相关的分子机制,能够更好地理解肾脏健康与疾病之间的关联,并为肾脏疾病的治疗和预防提供新的方向。运动能够增加PGC-1α的表达,并通过多种途径改善肾脏功能,进一步研究PGC-1α的活性调控对于开发肾脏疾病的治疗策略具有重要意义。PGC-1α在运动改善肾脏衰老中作用的相关研究进展见表4。
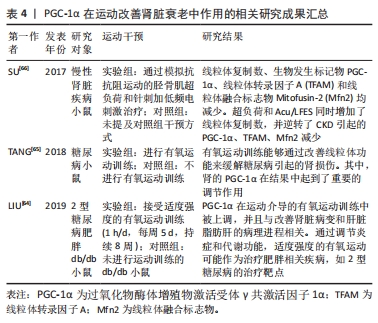
2.3.5 PGC-1α在运动改善骨骼肌衰老中的作用 骨骼肌在衰老过程中对于运动、机动性、能量平衡和新陈代谢起着至关重要的作用[6]。然而,随着衰老的发展,骨骼肌的功能和质量会下降,导致肌肉萎缩。研究发现,线粒体在这一退化过程中起着重要的调控作用,其中包括PGC-1α的调控作用[6]。
进一步的研究发现,无论是老年人还是年轻人,体育运动都有助于调节肌肉中PGC-1α的表达,并增强PGC-1α对线粒体健康的作用[67-68]。例如,KOLTAI等[69]的研究证明,有氧训练能够提高老年大鼠腓肠肌中PGC-1α通路中多个因子的蛋白含量,并增加线粒体生物发生的几个指标;另外,该运动方案还减少了老年动物与年轻动物之间其他与线粒体健康相关的分子差异[69]。另外,KANG等[17]证明了跑步机跑步能够增加腓肠肌中PGC-1α的含量,并上调其他与线粒体健康相关的因子。另外,耐力训练亦通过降低老年大鼠中特定分子的水平,进而预防肌肉凋亡[35]。然而,运动强度并不直接影响肌肉中PGC-1α蛋白的表达,这是由于运动强度与PGC-1α蛋白的表达水平并不存在明确的线性关系[70]。吴菊花等[71]的研究发现,低氧运动能够促进肥胖大鼠骨骼肌中PGC-1α的表达,从而改善肥胖大鼠骨骼肌的能量代谢。冯丽丽等[72]发现,有氧运动和抗阻运动能够促进骨骼肌中Sestrin 2蛋白、磷酸化AMP激活的蛋白激酶/AMP激活的蛋白激酶、PGC-1α的表达,其中抗阻运动的效果最好。
动物模型的研究揭示了肌肉对体育运动的复杂机制。通过对老年小鼠的研究发现,特异敲除或过表达PGC-1α都会对肌肉功能产生影
响[73]。具体而言,在肌肉中敲除PGC-1α时,脚踏车运动对氧化磷酸化蛋白的正面效果受到抑制;相反,PGC-1α的过表达增强了有氧运动对线粒体形态和氧化纤维比例的影响,并促进了运动能力和平衡的提升[73]。这些结果说明肌肉中PGC-1α在运动过程中具有重要作用,并且其水平受到运动方式和强度的调节。另外,细胞器更新在维持能量平衡中至关重要[73]。研究表明,PGC-1α的过表达能够调节衰老肌肉特有的线粒体自噬途径,并根据细胞代谢状态调整自噬[74]。有氧运动通过PGC-1α介导的相互作用抑制衰老引起的线粒体自噬的增加,在维持肌肉健康方面起到积极作用[75]。另外,肌肉能力的状态还能调节肝脏线粒体的自噬,并且PGC-1α在这两个器官之间起到信号传递的作用[74]。这些发现提示了线粒体自噬在肌肉功能和能量代谢中的重要性,并且通过调节PGC-1α
水平来调控自噬过程可能是改善肌肉衰老的潜在途径。研究还表明,高强度间歇性运动对核内PGC-1α蛋白水平的促进作用更为显著,而运动强度与PGC-1α mRNA的表达存在依赖关系[76]。总训练量对肌内PGC-1α蛋白含量的增长起着关键作用,而周期性的冲刺性间歇训练仍能刺激PGC-1α总蛋白的提高[76]。这些结果提示,通过适当的运动强度和训练量来调节PGC-1α的表达水平,可能是提高肌肉健康和逆转衰老效应的有效策略。
综上所述,通过运动调控骨骼肌中的PGC-1α能够改善衰老导致的肌肉退化和线粒体功能恶化。不同类型、时长和强度的运动都对PGC-1α的表达具有影响,而正确选择合适的运动方式和强度则有助于最大化PGC-1α在肌肉中的调节效果。
2.3.6 PGC-1α在运动改善肝脏老化中的作用 衰老过程中的肝病存在着线粒体结构和活性的变化,这会导致呼吸链中电子流的堵塞,进而引发线粒体反应性氧化物的生成,形成一个恶性循环[77]。为了维持肝脏的能量稳态,PGC-1α信号通路介导了线粒体氧化应激的调节,通过细胞器周转、蛋白聚集反应、线粒体自噬和线粒体生物发生来实现[7]。
PGC-1α在运动改善肝脏老化中具有重要作用。已有许多研究发现内质网应激与肝脏的线粒体功能障碍密切相关。Kristensen等[77]进行了一项实验,发现终身运动训练能够预防老年小鼠肝脏中免疫球蛋白蛋白、肌醇需求酶1α和蛋白激酶R样内质网激酶的异常变化,表明运动训练通过降低肌醇需求酶1α的水平来调节肝脏中蛋白质聚集反应。然而,这项实验的结果还表明,终身运动训练对蛋白质聚集反应的调节作用较小,但肌醇需求酶1α水平较低[77]。这些研究结果表明,PGC-1α在调节肝脏中诱导内质网应激的能力方面具有特定的调控作用。
另外一项研究发现,相对于有氧运动,抗阻运动训练能够更有效地激活成纤维细胞生长因子21信号通路下游信号因子PGC-1α和PGC-1β的表达,进一步促进成纤维细胞生长因子21的生理作用和脂肪酸氧化[78],这一发现进一步证明了PGC-1α在调节肝脏功能中的重要作用。其他研究亦证明有氧运动对老年小鼠的肝脏功能具有积极影响。例如,每周3次、20 min/次、速度20 cm/s、持续3个月的跑步机训练能够提高老年小鼠肝脏中的烟酰胺腺嘌呤二核苷酸水平、去乙酰化酶活性和核转录因子κB 去乙酰化水平,同时降低肝脏中PGC-1α的醋酸化水平[79]。
另外,耐力运动亦能降低去卵巢大鼠的高血糖和高游离脂肪酸水平,改善胰岛素水平,并上调肝脏中去乙酰化因子1和PGC-1α的蛋白表达[80]。这些研究结果进一步支持了运动对肝脏功能的积极影响。
马国栋等[81]的实验发现,耐力训练后再给予急性酒精摄入能够降低大鼠肝脏与血液中的一些指标,同时显著提高线粒体生物合成蛋白PGC-1α、核呼吸因子1、线粒体转录因子A和线粒体复合物ⅣmRNA的表达,这一研究结果亦进一步揭示了PGC-1α在肝脏功能调节中的重要性。另外,运动还能改善肝脏纤维化的情况。通过对大鼠进行运动训练,能够提高PGC-1α mRNA水平,减少胶原沉积,从而发挥抗纤维化的作用[82]。然而,需要进行更多的研究来深入理解肝脏中不折叠蛋白与PGC-1α之间复杂的相互作用。邵长专等[83]研究发现,运动能够纠正非酒精性脂肪肝引起的肝脏线粒体功能紊乱,减轻氧化应激,抑制线粒体质量控制系统重塑,从而起到保护作用,其中PGC-1α作为一个调控线粒体合成的重要因子,在非酒精性脂肪肝的发展中具有关键的作用。
总之,运动训练对肝脏老化和功能改善具有积极影响,其中PGC-1α在调节肝脏中的内质网应激、蛋白质聚集反应、脂肪酸氧化等方面发挥着重要的作用。然而,关于PGC-1α在肝脏老化中的具体调控机制还需要进一步研究探讨。另外,运动还能通过调节成纤维细胞生长因子21信号通路、改善肝脏纤维化、降低肝脏与血液中的指标等多种途径来促进肝脏功能的改善。然而,仍需进行更多的研究来深入理解肝脏中不折叠蛋白与PGC-1α之间复杂的相互作用。

| [1] OKA SI, SABRY AD, CAWLEY KM, et al. Multiple levels of PGC-1α dysregulation in heart failure. Front Cardiovasc Med. 2020;7:2. [2] KONG S, CAI B, NIE Q. PGC-1α affects skeletal muscle and adipose tissue development by regulating mitochondrial biogenesis. Mol Genet Genomics. 2022;297(3):621-633. [3] Di W, LV J, JIANG S, et al. PGC-1: the energetic regulator in cardiac metabolism. Curr Issues Mol Biol. 2018;28:29-46. [4] KUCZYNSKA Z, METIN E, LIPUT M, et al. Covering the role of PGC-1α in the nervous system. Cells. 2021;11(1):111. [5] DUMESIC PA, EGAN DF, Gut P, et al. An evolutionarily conserved uORF regulates PGC1α and oxidative metabolism in mice, flies, and bluefin tuna. Cell Metab. 2019;30(1):190-200.e6. [6] GREVENDONK L, CONNELL N J, McCrum C, et al. Impact of aging and exercise on skeletal muscle mitochondrial capacity, energy metabolism, and physical function. Nature communications. 2021;12(1):4773. [7] LEVEILLE M, BESSE-PATIN A, JOUVET N, et al. PGC-1α isoforms coordinate to balance hepatic metabolism and apoptosis in inflammatory environments. Mol Metab. 2020;34:72-84. [8] HALLING JF, PILEGAARD H. PGC-1α-mediated regulation of mitochondrial function and physiological implications. Appl Physiol Nutr Metab. 2020;45(9): 927-936. [9] RIUS-PEREZ S, TORRES-CUEVAS I, MILLAN I, et al. PGC-1α, inflammation, and oxidative stress: an integrative view in metabolism. Oxid Med Cell Longev. 2020;2020:1452696. [10] CHEN H, FAN W, HE H, et al. PGC-1: a key regulator in bone homeostasis. J Bone Miner Metab. 2022;40(1):1-8. [11] NETO IVS, PINTO AP, MUNOZ VR, et al. Pleiotropic and multi-systemic actions of physical exercise on PGC-1α signaling during the aging process. Ageing Res Rev. 2023;87:101935. [12] CHENG CF, KU HC, LIN H. PGC-1α as a pivotal factor in lipid and metabolic regulation. Int J Mol Sci. 2018;19(11):3447. [13] LEE G, UDDIN MJ, KIM Y, et al. PGC‐1α, a potential therapeutic target against kidney aging. Aging Cell, 2019;18(5):e12994. [14] CHRISTIANSEN D, MURPHY RM, BANGSBO J, et al. Increased FXYD1 and PGC-1α mRNA after blood flow-restricted running is related to fibre type-specific AMPK signalling and oxidative stress in human muscle. Acta Physiol (Oxf). 2018;223(2):e13045. [15] BAE JH, JO A, CHO SC, et al. Farnesol prevents aging-related muscle weakness in mice through enhanced farnesylation of Parkin-interacting substrate. Sci Transl Med. 2023;15(711):eabh3489. [16] GARCIA S, NISSANKA N, MARECO EA, et al. Overexpression of PGC‐1α in aging muscle enhances a subset of young‐like molecular patterns. Aging cell. 2018;17(2):e12707. [17] KANG J, WANG Y, WANG D. Endurance and resistance training mitigate the negative consequences of depression on synaptic plasticity through different molecular mechanisms. Int J Neurosci. 2020;130(6):541-550. [18] MONTORI-GRAU M, AGUILAR-RECARTE D, ZAREI M, et al. Endoplasmic reticulum stress downregulates PGC-1α in skeletal muscle through ATF4 and an mTOR-mediated reduction of CRTC2. Cell Commun Signal. 2022;20(1):53. [19] YE L, LI M, WANG Z, et al. Depression of mitochondrial function in the rat skeletal muscle model of myofascial pain syndrome is through down-regulation of the AMPK-PGC-1α-SIRT3 axis. J Pain Res. 2020;13:1747-1756. [20] YIN F, ZHANG J, LIU Y, et al. Basolateral amygdala SIRT1/PGC-1α mitochondrial biogenesis pathway mediates morphine withdrawal-associated anxiety in mice. Int J Neuropsychopharmacol. 2022;25(9):774-785. [21] 张丽,何俊,金虹,等.HO-1/PGC-1α通路在调控线粒体氧化应激中的作用[J].中国药理学通报,2022,38(8):1137-1141. [22] SHI Y, SHU ZJ, WANG H, et al. Altered expression of hepatic β-adrenergic receptors in aging rats: implications for age-related metabolic dysfunction in liver. Am J Physiol Regul Integr Comp Physiol. 2018;314(4):R574-R583. [23] DELRIO-LORENZO A, ROJO-RUIZ J, ALONSO MT, et al. Sarcoplasmic reticulum Ca2+ decreases with age and correlates with the decline in muscle function in Drosophila. J Cell Sci. 2020;133(6):jcs240879. [24] LI W, CAO J, WANG X, et al. Ferruginol restores SIRT1-PGC-1α-mediated mitochondrial biogenesis and fatty acid oxidation for the treatment of DOX-induced cardiotoxicity. Front Pharmacol. 2021;12:773834. [25] ZHU Z, HU J, CHEN Z, et al. Transition of acute kidney injury to chronic kidney disease: role of metabolic reprogramming. Metabolism. 2022;131:155194. [26] WONDMKUN YT. Obesity, insulin resistance, and type 2 diabetes: associations and therapeutic implications. Diabetes Metab Syndr Obes. 2020;13:3611-3616. [27] FOREMAN NA, HESSE AS, JI LL. Redox signaling and sarcopenia: searching for the primary suspect. Int J Mol Sci. 2021;22(16):9045. [28] ABU SHELBAYEH O, ARROUM T, MORRIS S, et al. PGC-1α is a master regulator of mitochondrial lifecycle and ROS stress response. Antioxidants. 2023;12(5):1075. [29] FU H, LIU H. Deletion of toll-like receptor 4 ameliorates diabetic retinopathy in mice. Arch Phys Biochem. 2023;129(2):519-525. [30] HUANG DD, FAN SD, CHEN XY, et al. Nrf2 deficiency exacerbates frailty and sarcopenia by impairing skeletal muscle mitochondrial biogenesis and dynamics in an age-dependent manner. Exp Gerontol. 2019;119:61-73. [31] KELEHER MR, ERICKSON K, SMITH HA, et al. Placental insulin/IGF-1 signaling, PGC-1α, and inflammatory pathways are associated with metabolic outcomes at 4–6 years of age: the ECHO healthy start cohort. Diabetes. 2021;70(3): 745-751. [32] XU H, DU X, XU J, et al. Pancreatic β cell microRNA-26a alleviates type 2 diabetes by improving peripheral insulin sensitivity and preserving β cell function. PLoS Biol. 2020;18(2):e3000603. [33] BESSE-PATIN A, JEROMSON S, LEVESQUE-DAMPHOUSSE P, et al. PGC1A regulates the IRS1: IRS2 ratio during fasting to influence hepatic metabolism downstream of insulin. Proc Natl Acad Sci U S A. 2019;116(10):4285-4290. [34] SEKSARIA S, MEHAN S, DUTTA B J, et al. Oxymatrine and insulin resistance: Focusing on mechanistic intricacies involve in diabetes associated cardiomyopathy via SIRT1/AMPK and TGF‐β signaling pathway. J Biochem Mol Toxicol. 2023;37(5):e23330. [35] 王少堃,王世强,王一杰,等.骨骼肌介导的运动神经保护效应:作用途径和分子机制[J].中国体育科技,2023,59(4):58-66. [36] KIM SB, HEO JI, KIM H, et al. Acetylation of PGC1α by histone deacetylase 1 downregulation is implicated in radiation-induced senescence of brain endothelial cells. J Gerontol A Biol Sci Med Sci. 2019;74(6):787-793. [37] ZIA A, SAHEBDEL F, FARKHONDEH T, et al. A review study on the modulation of SIRT1 expression by miRNAs in aging and age-associated diseases. Int J Biol Macromol. 2021;188:52-61. [38] MAHBOOBIFARD F, POURGHOLAMI MH, JORJANI M, et al. Estrogen as a key regulator of energy homeostasis and metabolic health. Biomed Pharmacother. 2022;156:113808. [39] CANTO C, GERHART-HINES Z, FEIGE JN, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458(7241):1056-1060. [40] OU MY, ZHANG H, TAN PC, et al. Adipose tissue aging: mechanisms and therapeutic implications. Cell Death Dis. 2022;13(4):300. [41] MACEDO APA, DA SILVA ASR, MUNOZ VR, et al. Mitochondrial dysfunction plays an essential role in remodeling aging adipose tissue. Mech Ageing Dev. 2021;200:111598. [42] ZIEGLER AK, DAMGAARD A, MACKEY AL, et al. An anti-inflammatory phenotype in visceral adipose tissue of old lean mice, augmented by exercise. Sci Rep. 2019;9(1):12069. [43] THIRUPATHI A, DA SILVA PIERI BL, QUEIROZ JAMP, et al. Strength training and aerobic exercise alter mitochondrial parameters in brown adipose tissue and equally reduce body adiposity in aged rats. J Physiol Biochem. 2019;75(1): 101-108. [44] SUN L, LI FH, HAN C, et al. Alterations in mitochondrial biogenesis and respiratory activity, inflammation of the senescence-associated secretory phenotype, and lipolysis in the perirenal fat and liver of rats following lifelong exercise and detraining. FASEB J. 2021;35(10):e21890. [45] 翁锡全,王朝格,林宝璇,等.低温下运动对肥胖大鼠白色脂肪棕色化及相关调节因子表达的影响[J].中国运动医学杂志,2021,40(1):38-45. [46] 付鹏宇,龚丽景,段佳妍,等.低氧运动对肥胖小鼠脂肪UCP-1和PGC-1α表达的影响[J].中国运动医学杂志,2015,34(11):1070-1075. [47] BAYOD S, DEL VALLE J, LALANZA JF, et al. Long-term physical exercise induces changes in sirtuin 1 pathway and oxidative parameters in adult rat tissues. Exp Gerontol. 2012;47(12):925-935. [48] CHEN WK, TSAI YL, SHIBU MA, et al. Exercise training augments Sirt1-signaling and attenuates cardiac inflammation in D-galactose induced-aging rats. Aging (Albany NY). 2018;10(12):4166-4174. [49] YEO HS, LIM JY. Effects of different types of exercise training on angiogenic responses in the left ventricular muscle of aged rats. Exp Gerontol. 2022; 158:111650. [50] GU Q, WANG B, ZHANG XF, et al. Chronic aerobic exercise training attenuates aortic stiffening and endothelial dysfunction through preserving aortic mitochondrial function in aged rats. Exp Gerontol. 2014;56:37-44. [51] GIOSCIA-RYAN RA, BATTSON ML, CUEVAS LM, et al. Voluntary aerobic exercise increases arterial resilience and mitochondrial health with aging in mice. Aging (Albany NY). 2016;8(11):2897-2914. [52] MAHDAVI N, JOUKAR S, NAJAFIPOUR H, et al. Promotion of aging heart function and its redox balance following hind-limb blood flow restriction plus endurance exercise training in rats: klotho and PGC1-α as involving candidate molecules. Pflugers Arch. 2022;474(7):699-708. [53] BOTTA A, LAHER I, BEAM J, et al. Short term exercise induces PGC-1α, ameliorates inflammation and increases mitochondrial membrane proteins but fails to increase respiratory enzymes in aging diabetic hearts. PLoS One. 2013;8(8):e70248. [54] MCMEEKIN LJ, FOX SN, BOAS SM, et al. Dysregulation of PGC-1α-dependent transcriptional programs in neurological and developmental disorders: therapeutic challenges and opportunities. Cells. 2021;10(2):352. [55] LIN JY, KUO WW, BASKARAN R, et al. Swimming exercise stimulates IGF1/ PI3K/Akt and AMPK/SIRT1/PGC1α survival signaling to suppress apoptosis and inflammation in aging hippocampus [published correction appears in Aging (Albany NY). Aging (Albany NY). 2020;12(8):6852-6864. [56] E L, BURNS JM, SWERDLOW RH. Effect of high-intensity exercise on aged mouse brain mitochondria, neurogenesis, and inflammation. Neurobiol Aging. 2014;35(11):2574-2583. [57] GUSDON AM, CALLIO J, DISTEFANO G, et al. Exercise increases mitochondrial complex I activity and DRP1 expression in the brains of aged mice. Exp Gerontol. 2017;90:1-13. [58] KARLSSON L, GONZALEZ-ALVARADO MN, MOTALLEB R, et al. Constitutive PGC-1α overexpression in skeletal muscle does not contribute to exercise-induced neurogenesis. Mol Neurobiol. 2021;58(4):1465-1481. [59] CHEN Z, YUAN Z, YANG S, et al. Brain energy metabolism: astrocytes in neurodegenerative diseases. CNS Neurosci Ther. 2023;29(1):24-36. [60] SVENSSON K, SCHNYDER S, CARDEL B, et al. Loss of renal tubular PGC-1α exacerbates diet-induced renal steatosis and age-related urinary sodium excretion in mice. PLoS One. 2016;11(7):e0158716. [61] TRAN MT, ZSENGELLER ZK, BERG AH, et al. PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature. 2016;531(7595): 528-532. [62] YUAN L, YUAN Y, LIU F, et al. PGC-1α alleviates mitochondrial dysfunction via TFEB-mediated autophagy in cisplatin-induced acute kidney injury. Aging (Albany NY). 2021;13(6):8421-8439. [63] HAN SH, WU MY, NAM BY, et al. PGC-1α protects from notch-induced kidney fibrosis development. J Am Soc Nephrol. 2017;28(11):3312-3322. [64] LIU HW, KAO HH, WU CH. Exercise training upregulates SIRT1 to attenuate inflammation and metabolic dysfunction in kidney and liver of diabetic db/db mice. Nutr Metab (Lond). 2019;16:22. [65] TANG LX, WANG B, WU ZK. Aerobic exercise training alleviates renal injury by interfering with mitochondrial function in type-1 diabetic mice. Med Sci Monit. 2018;24:9081-9089. [66] SU Z, KLEIN JD, DU J, et al. Chronic kidney disease induces autophagy leading to dysfunction of mitochondria in skeletal muscle. Am J Physiol Renal Physiol. 2017;312(6):F1128-F1140. [67] RIBEIRO MBT, GUZZONI V, HORD JM, et al. Resistance training regulates gene expression of molecules associated with intramyocellular lipids, glucose signaling and fiber size in old rats. Sci Rep. 2017;7(1):8593. [68] HUANG CC, WANG T, TUNG YT, et al. Effect of exercise training on skeletal muscle SIRT1 and PGC-1α expression levels in rats of different age. Int J Med Sci. 2016;13(4):260-270. [69] KOLTAI E, HART N, TAYLOR AW, et al. Age-associated declines in mitochondrial biogenesis and protein quality control factors are minimized by exercise training. Am J Physiol Regul Integr Comp Physiol. 2012;303(2):R127-R134. [70] 许杰,黄巧婷,谢敏豪,等.不同强度运动对大鼠骨骼肌AMPK/PGC-1α信号通路的影响[J].成都体育学院学报,2018,44(4):121-126. [71] 吴菊花,杨亚南,翁锡全,等.低氧运动干预营养性肥胖模型大鼠骨骼肌能量代谢的变化[J].中国组织工程研究,2022,26(29):4598-4604. [72] 冯丽丽,李博文,田振军.运动激活SESN2/AMPK/PGC-1α通路改善心梗诱导的骨骼肌减少[J].北京体育大学学报,2021,44(5):128-137. [73] GILL JF, SANTOS G, SCHNYDER S, et al. PGC-1α affects aging-related changes in muscle and motor function by modulating specific exercise-mediated changes in old mice. Aging Cell. 2018;17(1):e12697. [74] CHRISTENSEN NM, RINGHOLM S, BUCH BT, et al. Muscle PGC-1α modulates hepatic mitophagy regulation during aging. Exp Gerontol. 2023;172:112046. [75] KIM Y, TRIOLO M, HOOD D A. Impact of aging and exercise on mitochondrial quality control in skeletal muscle. Oxid Med Cell Longev. 2017;2017:3165396. [76] 殷彰冶,郜卫峰.耐力运动调控青壮年健康人群骨骼肌PGC-1α表达研究进展[J].中国运动医学杂志,2022,41(9):725-736. [77] KRISTENSEN CM, BRANDT CT, RINGHOLM S, et al. PGC-1α in aging and lifelong exercise training-mediated regulation of UPR in mouse liver. Exp Gerontol. 2017;98:124-133. [78] 李良,徐建方,冯连世,等.有氧运动和抗阻运动训练对肥胖大鼠肝脏FGF21信号通路的影响[J].中国运动医学杂志,2018,37(10):847-856. [79] BIANCHI A, MARCHETTI L, HALL Z, et al. Moderate exercise inhibits age-related inflammation, liver steatosis, senescence, and tumorigenesis. J Immunol. 2021;206(4):904-916. [80] 陈晓光,徐晓阳,薛博洋,等.耐力运动对去卵巢大鼠肝脏SIRT1和PGC-1α蛋白表达的影响[J].北京体育大学学报,2013,36(4):62-67. [81] 马国栋,董杰,刘艳环.耐力训练预防急性酒精性肝损伤机制:线粒体生物合成[J].上海体育学院学报,2012,36(1):73-77. [82] KHODABANDEH M, PEERI M, AZARBAYJANI MA, et al. Effect of resistance exercise and liposomal vitamin C on some factors of mitochondrial dynamics and biogenesis. Complement Med J. 2021;11(1):82-97. [83] 邵长专,江红轲.耐力训练纠正高脂饮食相关的非酒精性脂肪肝大鼠肝脏线粒体功能紊乱探讨[J].山东体育学院学报,2019,35(2):73-81. |
| [1] | 吴 菁, 姚英策, 杨晓巍, 薛博士, 赵建斌, 杨 辰, 栾天峰, 周志鹏. 肌力训练与神经肌肉电刺激干预髌股关节痛患者下肢功能和生物力学的变化[J]. 中国组织工程研究, 2024, 28(9): 1365-1371. |
| [2] | 余伟杰, 刘爱峰, 陈继鑫, 郭天赐, 贾易臻, 冯汇川, 杨家麟. 机器学习在腰椎间盘突出症诊治中的优势和应用策略[J]. 中国组织工程研究, 2024, 28(9): 1426-1435. |
| [3] | 杨玉芳, 杨芷姗, 段棉棉, 刘毅恒, 唐正龙, 王 宇. 促红细胞生成素在骨组织工程中的应用及前景[J]. 中国组织工程研究, 2024, 28(9): 1443-1449. |
| [4] | 陈凯佳, 刘景云, 曹 宁, 孙建波, 周 燕, 梅建国, 任 强. 组织工程技术在股骨头坏死治疗中的应用及前景[J]. 中国组织工程研究, 2024, 28(9): 1450-1456. |
| [5] | 王 继, 张 敏, 李文博, 杨中亚, 张 龙. 有氧运动对2型糖尿病大鼠糖脂代谢、骨骼肌炎症和自噬的影响[J]. 中国组织工程研究, 2024, 28(8): 1200-1205. |
| [6] | 刘 鑫, 胡 满, 赵文杰, 张 钰, 孟 博, 杨 盛, 彭 晴, 张 亮, 王静成. 镉暴露激活PI3K/Akt信号通路诱导椎间盘纤维环细胞衰老[J]. 中国组织工程研究, 2024, 28(8): 1217-1222. |
| [7] | 周邦瑜, 李 杰, 阮玉山, 耿福能, 李绍波. 美洲大蠊研粉干预脊髓半横断大鼠运动功能和自噬蛋白Beclin-1的表达[J]. 中国组织工程研究, 2024, 28(8): 1223-1228. |
| [8] | 阮 蓉, 娄旭佳, 金其贯, 章立冰, 徐 尚, 胡玉龙. 白藜芦醇可调控运动性疲劳大鼠的糖异生[J]. 中国组织工程研究, 2024, 28(8): 1229-1234. |
| [9] | 娄 国, 张 艳, 付常喜. 内皮型一氧化氮合酶在运动预适应改善心肌缺血-再灌注损伤中的作用[J]. 中国组织工程研究, 2024, 28(8): 1283-1288. |
| [10] | 林泽玉, 徐 林. 痛风致骨破坏机制的研究与进展[J]. 中国组织工程研究, 2024, 28(8): 1295-1300. |
| [11] | 王伟庆, 周 越. 慢性炎症调控脂肪组织的纤维化[J]. 中国组织工程研究, 2024, 28(8): 1307-1312. |
| [12] | 梅静怡, 刘 江, 肖 聪, 刘 鹏, 周浩浩, 林展翼. 组织工程血管构建过程中平滑肌细胞增殖变化及代谢模式[J]. 中国组织工程研究, 2024, 28(7): 1043-1049. |
| [13] | 王姗姗, 舒 晴, 田 峻. 物理因子促进干细胞的成骨分化[J]. 中国组织工程研究, 2024, 28(7): 1083-1090. |
| [14] | 潘小龙, 樊飞燕, 应春苗, 刘飞祥, 张运克. 中药抑制间充质干细胞衰老的作用及机制[J]. 中国组织工程研究, 2024, 28(7): 1091-1098. |
| [15] | 徐灿丽, 何文星, 汪 磊, 吴芳婷, 王佳慧, 段雪琳, 赵铁建, 赵 斌, 郑 洋. 肝脏类器官研究的文献计量学分析[J]. 中国组织工程研究, 2024, 28(7): 1099-1104. |
当前,已有部分综述回顾了PGC-1α在衰老中的作用以及运动的调控作用[8-9]。然而,这些综述涉及的层面只停留在衰老的宏观概念上,缺乏对PGC-1α在人体不同组织和器官中调控情况的具体回顾。这使得运动对PGC-1α在不同组织和器官中调控情况及其抗衰老的具体机制尚不清楚。因此,该综述旨在基于不同人体组织和器官的视角,详细回顾PGC-1α在运动抗衰老中的作用。 中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
1.1.1 检索人及检索时间 第一、二、三作者于2023-05-01/07-01进行文献检索。
1.1.2 检索文献时限 各数据库建库至2023年9月。
1.1.3 检索数据库 Web of Science、PubMed、中国知网、万方和维普数据库。
1.1.4 检索词 中文检索词为“PGC-1α,PGC-1alpha,PPARGC1A,衰老,老化,运动,体育活动,运动训练,运动治疗,有氧运动,心血管,有氧运动,氧气耗尽,心血管运动,抗阻训练,肌力训练,重力训练,抗阻力训练,力量训练,脂肪组织,脂肪细胞,脂肪代谢,脂代谢,心血管,心血管疾病,心血管健康,心血管系统,肝脏,肝脏功能,肝脏疾病,神经系统,神经元,神经功能,神经疾病,肾脏,肾功能,肾脏疾病,肾脏健康,骨骼肌,肌肉,骨骼肌功能,肌肉功能,肌肉健康”;英文检索词为“PGC-1α,PGC-1 alpha,PPARGC1A,aging,senescence,exercise,exercise training,aerobic exercise,endurance training,resistance training,adipose tissue,cardiovascular,liver,nervous system,kidneys,skeletal muscles”。
1.1.5 检索策略 运用布尔逻辑运算符“OR”和“AND”分别将检索词连接进行检索。以PubMed和中国知网数据库为例,文献检索的详细策略见图1。
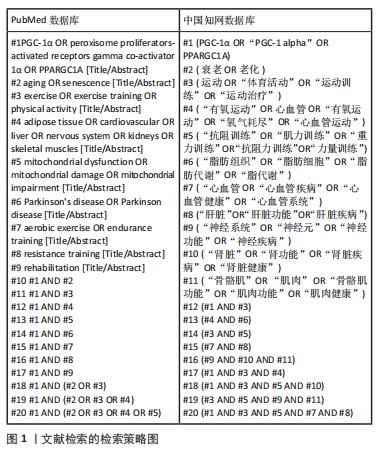
1.2 纳入和排除标准
1.2.1 纳入标准 ①随机对照试验、单因素试验、综述、病例报告和非随机性历史对照试验等;②研究内容为PGC-1α在运动抗衰老中的作用文献;③经同行专家评审认可并发表的文献;④期刊文献发表时期刊被中国科技论文统计源期刊、北京大学中文核心期刊目录、中国科学引文数据库(含扩展版)、中国社会科学引文索引(含扩展版)、科学引文索引(含扩展版)、工程索引(含扩展版)和PubMed等一个或多个数据库收录。
1.2.2 排除标准 ①研究内容和此次综述主题不相关的文献;②所在期刊无同行评审环节的文献;③学位论文;④重复性研究和低质量文献;⑤纳入相应的文献不符合SYRCLE评价工具(评估动物模型研究)、
Cochrane工具(评估临床随机对照试验)以及AMSTAR 2量表(评估系统评价和Meta分析)中规定的条目。
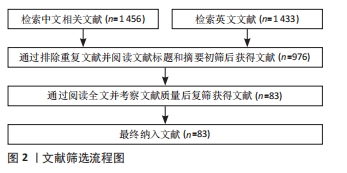
1.3 文献质量评价和数据的提取 数据库共检索到文献2 889篇,严格按照纳入和排除标准进行筛选,最终纳入文献83篇,包括英文文献71篇,其中Web of Science和PubMed均71篇(重复);中文文献12篇,其中中国知网、万方及维普数据库均12篇(重复)。文献筛选流程图见图2。
3.2 作者综述区别于他人他篇的特点 该综述在以往研究的基础上,从不同人体组织和器官的视角出发,详细回顾了PGC-1α在运动抗衰老中的作用。相较于以往的综述,该综述着重关注了PGC-1α在不同组织和器官中的调控情况,并探讨了不同类型、强度和时长的运动对PGC-1α表达的调节影响。此外,文章对于PGC-1α的蛋白修饰和表观遗传变化进行了详细讨论,探索了这些调控机制在衰老过程中的潜在作用。因此,该综述在深入挖掘PGC-1α在运动抗衰老中的作用机制方面具有独特的特点。
3.3 综述的局限性 尽管该综述对PGC-1α在运动抗衰老中的作用进行了深入探讨,但仍存在一些局限性。首先,由于研究范围和篇幅的限制,文章未能对每种类型、强度和时长的运动对PGC-1α的调节影响进行详尽讨论,这是需要在未来的研究中进一步探索的问题。其次,对于PGC-1α在运动抗衰老中的具体机制,文章在一定程度上停留在总结性的概念上,未能对一些细节问题进行深入研究,有待后续的实验和理论研究加以解答。
3.4 综述的重要意义 该综述通过对PGC-1α在不同组织和器官中的调控情况进行深入回顾,以及综合讨论了运动对PGC-1α表达的调节作用,有助于更好地理解PGC-1α在衰老调控中的作用机制。此外,通过对PGC-1α的蛋白修饰和表观遗传变化的讨论,该综述为进一步探索PGC-1α的功能调节机制和开发针对衰老相关疾病的治疗策略提供了重要参考。因此,该综述的重要意义在于为PGC-1α的研究提供了新的思路和方向。
3.5 课题专家组对未来的建议 基于对PGC-1α在运动抗衰老中的调控作用的综合讨论,课题专家组对未来的研究提出以下建议:①进一步探索不同类型、强度和时长的运动对PGC-1α表达的调节影响,深入研究最佳的运动模式以及运动对PGC-1α的剂量效应;②深入探索PGC-1α的蛋白修饰和表观遗传变化的调节机制及其在衰老过程中的作用,有针对性地开展实验研究,进一步揭示PGC-1α的功能调节机制;③加强对PGC-1α在不同衰老相关疾病中的作用机制的研究,探索PGC-1α作为治疗靶点的潜在价值,为开发治疗策略提供更可靠的理论基础。 中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
 #br#
#br#
文题释义:
PGC-1α:中文全称为过氧化物酶体增殖物激活受体γ共激活因子1α,是一种转录共激活因子,主要在肝脏、肌肉和脑等组织中表达,能够调节细胞能量代谢和线粒体功能,并参与调节血糖代谢、脂肪酸氧化和肌肉适应性等生物学过程,其功能受多种生理和环境因素的调控,包括运动、饥饿、冷暴露和泌乳酸。衰老:是人类或动物体内发生的一系列生理过程,表现为身体功能逐渐下降、肌肉减少、皮肤变得松弛、免疫系统减弱、记忆力下降等。随着年龄的增长,人体的各种系统逐渐失去原有的功能和弹性,导致身体功能减弱,容易受到疾病的侵袭。衰老是不可逆转的过程,但通过健康的生活方式和医学干预可以延缓衰老的进程。
该综述基于不同人体组织和器官的视角,详细回顾了PGC-1α在运动抗衰老中的作用。主要内容包括PGC-1α的定义和功能、PGC-1α与衰老的关系,以及PGC-1α在运动改善不同组织和器官衰老中的作用。通过这样的深入研究,能够更好地理解PGC-1α在衰老调控中的作用机制,并为在不同组织和器官中寻找抗衰老和预防相关疾病的干预策略提供新思路和指导。
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||