中国组织工程研究 ›› 2024, Vol. 28 ›› Issue (31): 5017-5021.doi: 10.12307/2024.718
• 干细胞基础实验 basic experiments of stem cells • 上一篇 下一篇
两种供试品不同浸提条件及作用剂量下的体外人淋巴细胞增殖实验
许建霞,付海洋,曲守方
- 中国食品药品检定研究院,北京市 102629
In vitro human lymphocyte proliferation assay under different extraction conditions and doses of two types of test samples
Xu Jianxia, Fu Haiyang, Qu Shoufang
- National Institutes for Food and Drug Control, Beijing 102629, China
摘要:
文题释义:
体外淋巴细胞增殖实验:又称淋巴细胞转化实验,是指体外培养的T淋巴细胞在有丝分裂原或特异性抗原的刺激下发生免疫应答,细胞的数量或代谢活性会增加。淋巴细胞增殖实验被广泛应用于药物过敏研究,在动物源性医疗器械的免疫原性研究中也占有重要地位,属于较为敏感的功能性检验方法。植物血凝素:是发现于植物特别是豆科植物中的凝集素,属于高分子糖蛋白类,是低聚糖和蛋白质的复合物,具有促进有丝分裂的活性。
背景:体外淋巴细胞增殖实验常用于检测医疗器械潜在的免疫原性,但在相关标准中均未给出详尽的浸提条件及作用剂量。
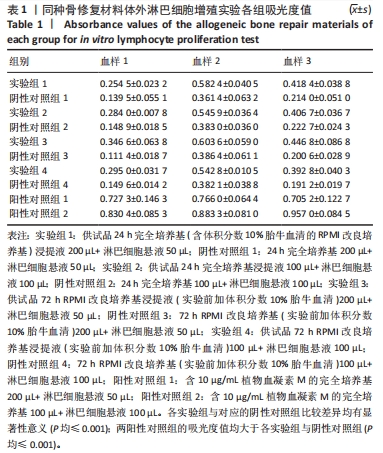
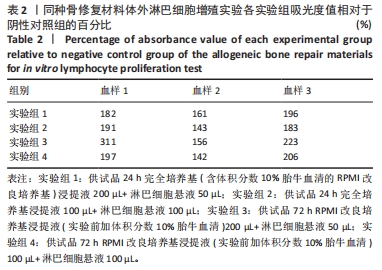
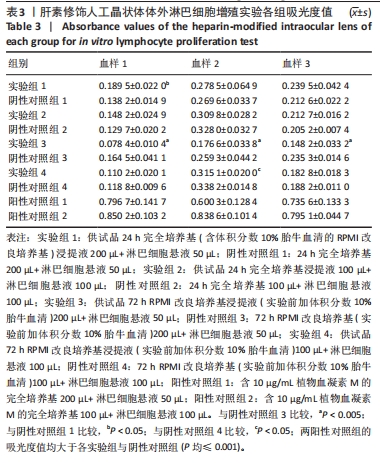
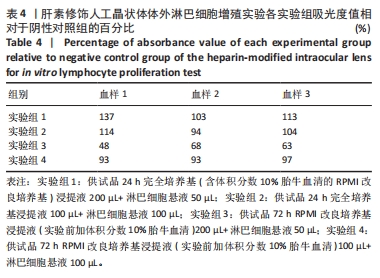
目的:考察供试品不同浸提条件及作用剂量对体外人淋巴细胞增殖的影响,思考在选择体外淋巴细胞增殖实验条件时需考虑的因素。方法:实验检测同种骨修复材料与肝素修饰人工晶状体两种供试品,均分为以下12组:①实验组1:供试品24 h完全培养基(含体积分数10%胎牛血清的RPMI改良培养基)浸提液200 µL+淋巴细胞悬液50 µL;②阴性对照组1:24 h完全培养基200 µL+淋巴细胞悬液50 µL;③实验组2:供试品24 h完全培养基浸提液100 µL+淋巴细胞悬液100 µL;④阴性对照组2:24 h完全培养基100 µL+淋巴细胞悬液100 µL;⑤实验组3:供试品72 h RPMI改良培养基浸提液(实验前加体积分数10%胎牛血清)200 µL+淋巴细胞悬液50 µL;⑥阴性对照组3:72 h RPMI改良培养基(实验前加体积分数10%胎牛血清)200 µL+淋巴细胞悬液50 µL;⑦实验组4:供试品72 h RPMI改良培养基浸提液(实验前加体积分数10%胎牛血清)100 µL+淋巴细胞悬液100 µL;⑧阴性对照组4:72 h RPMI改良培养基(实验前加体积分数10%胎牛血清)100 µL+淋巴细胞悬液100 µL;⑨阳性对照组1:含10 μg/mL植物血凝素M的完全培养基200 µL+淋巴细胞悬液50 µL;⑩阳性对照组2:含10 μg/mL植物血凝素M的完全培养基100 µL+淋巴细胞悬液100 µL;⑪空白对照组1:250 µL完全培养基;⑫空白对照组2:200 µL完全培养基。培养3 d后,采用CCK-8法检测淋巴细胞增殖。
结果与结论:①不同实验条件下,同种骨修复材料浸提液均可增强人淋巴细胞的活性,以RPMI改良培养基浸提72 h、浸提液与淋巴细胞悬液的体积比为4∶1的实验条件最为显著;肝素修饰人工晶状体在该条件下对淋巴细胞活性有明显的抑制作用,可能与浸提液中的肝素有关,但在完全培养基浸提24 h、浸提液与淋巴细胞悬液的体积比为4∶1的实验条件下对淋巴细胞活性有轻微的增强作用;②供试品不同浸提条件及作用剂量下,人体外淋巴细胞增殖实验结果可能会有较大差异,实验条件的选择需结合产品临床应用情况,也需考虑产品的固有特性。
https://orcid.org/0000-0003-4883-543X (许建霞)
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中图分类号: