中国组织工程研究 ›› 2013, Vol. 17 ›› Issue (16): 2874-2882.doi: 10.3969/j.issn.2095-4344.2013.16.004
• 组织工程骨及软骨材料 tissue-engineered bone and cartilage materials • 上一篇 下一篇
煅烧骨的安全性
刘斌钰1,郭敏芳1,邢雁霞1,马存根2, 3
- 1山西大同大学医学院,山西省大同市 037009;2山西大同大学脑科研究所,山西省大同市 037009;3山西中医学院,山西省太原市 030001
-
出版日期:2013-04-16发布日期:2013-04-16 -
通讯作者:马存根,教授,博士生导师,山西中医学院,山西省太原市 030001 -
作者简介:刘斌钰★,男,1976年生,山西省大同市人,硕士,副教授,主要从事骨组织工程的研究及口腔颌面外科的科研与临床研究。 -
基金资助:山西省高校科技研究开发项目(20111120)。
Safety of true bone ceramics
Liu Bin-yu1, Guo Min-fang1, Xing Yan-xia1, Ma Cun-gen2, 3.
- 1 Medical School, Shanxi Datong University, Datong 037009, Shanxi Province, China
2 Institute of Brain Science, Shanxi Datong University, Datong 037009, Shanxi Province, China
3 Shanxi University of Traditional Chinese Medicine, Taiyuan 030001, Shanxi Province, China
-
Online:2013-04-16Published:2013-04-16 -
Contact:Ma Cun-gen, Professor, Doctoral supervisor, Institute of Brain Science, Shanxi Datong University, Datong 037009, Shanxi Province, China; Shanxi University of Traditional Chinese Medicine, Taiyuan 030001, Shanxi Province, China -
About author:Liu Bin-yu★, Master, Associate professor, Medical School, Shanxi Datong University, Datong 037009, Shanxi Province, China liudaifu775@163.com -
Supported by:Shanxi University Technology Research and Development Projects, No. 20111120*
摘要:
背景:经高温处理的煅烧骨具有类似自然骨的连续微孔结构,良好的生物相容性和降解性。 目的:观察牛煅烧骨的生物相容性、细胞相容性及毒性。 方法:①细胞相容性实验:将牛煅烧骨与第3 代已诱导的Wistar大鼠骨髓间充质干细胞复合培养。②溶血实验:将煅烧骨浸提液、生理盐水与双蒸水加入兔血中。③凝血实验:将煅烧骨加入兔血浆中。④急性毒性实验:在昆明种小鼠尾静脉分别注射煅烧骨浸提液、生理盐水。⑤微核实验:在小鼠腹腔分别注射煅烧骨浸提液、生理盐水与环磷酰胺。⑥局部刺激性实验:将煅烧骨浸提液、生理盐水分别注射于兔两侧脊柱皮下。⑦热源检测实验:在兔耳静脉注射煅烧骨浸提液。⑧皮下植入实验:将煅烧骨材料植入Wistar大鼠背部皮下。 结果与结论:煅烧骨材料无细胞毒性,具有良好的细胞及血液相容性;对皮肤、肌肉无刺激作用;对心、肝、肾重要器官无毒性作用;皮下植入后对周围组织无刺激作用,能够部分降解吸收并被机体组织替代;无致热作用,对凝血功能无影响,对小鼠骨髓细胞无抑制及毒性作用。
中图分类号:
引用本文
刘斌钰,郭敏芳,邢雁霞,马存根.. 煅烧骨的安全性[J]. 中国组织工程研究, 2013, 17(16): 2874-2882.
Liu BY, Guo MF, Xing YX, Ma CG. Safety of true bone ceramics[J]. Chinese Journal of Tissue Engineering Research, 2013, 17(16): 2874-2882.
Bovine cancellous bone was white (Figure 1). The special fluorescent character of hydroxyapatite was found. The trabecular pattern presented the full structure, and the trabecular gap was the interconnected pore. The three-dimensional mesh structure was observed by electron microscope and the pore diameter was unequal 190-750 μm, the porosity was (80.39±1.40)%. On one hand, the connective mesh structure will benefit to the formation of osteon, which could provide larger surface for the proliferation of osteoblasts. On the other hand, it will benefit to the infiltration of nutritional ingredient and formation of vascular and degradation of materials.
Morphology of true bone ceramic-bone marrow mesenchymal stem cells complex

Observation under invert microscope
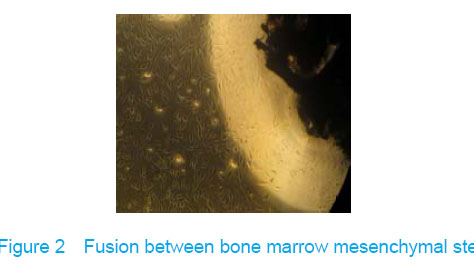
True bone ceramic mesh was filled with bone marrow mesenchymal stem cells immediately after bone marrow mesenchymal stem cells inoculated into the true bone ceramic, lots of cells adhered to the bracket after observed for 24 hours, and large extracellular matrix was secreted after 7 days. There was no clear boundary between the cells and the matrix, which indicated that there had fine fusion between the cell and the true bone ceramic (Figure 2).
Observation with scanning electron microscope
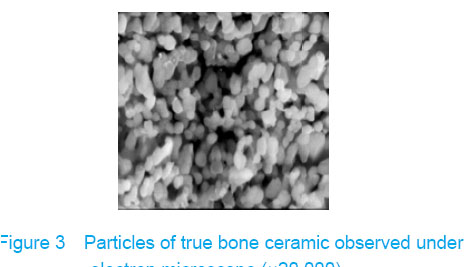
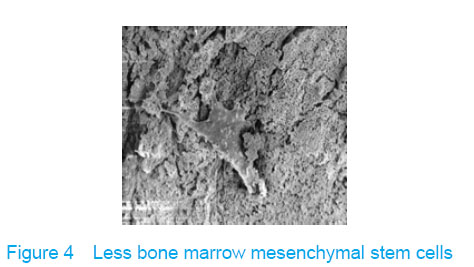
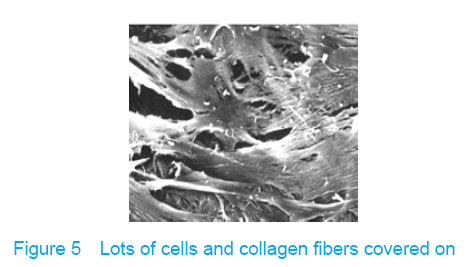
Electron microscope showed that a few cells adhered to true bone ceramic at 1 day after bone marrow mesenchymal stem cells incubation, and lots of cells and collagen fibers covered on the scaffold at 7 days and embedded scaffold basically (Figures 3-5).
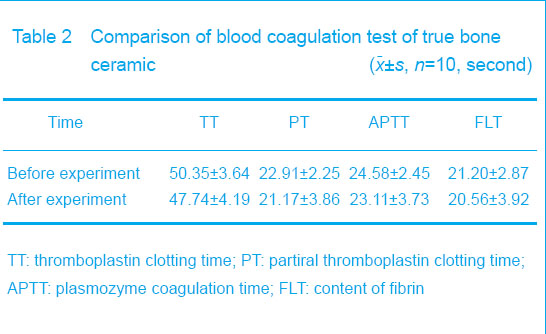
Blood coagulation test
Blood coagulation test showed that there were no changes in plasmozyme coagulation time, partiral thromboplastin clotting time, thromboplastin clotting time
and the content of fibrin before and after the experiment (P > 0.05), which indicated that the materials could not change the coagulation function (Table 2).
Test of subcutaneous implantation
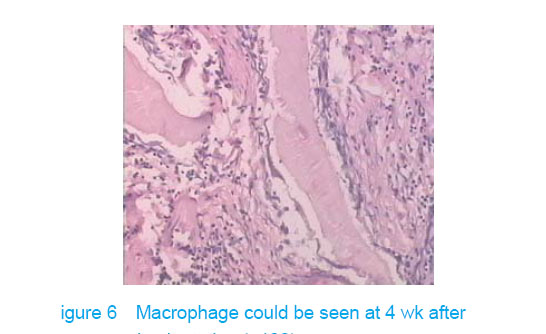
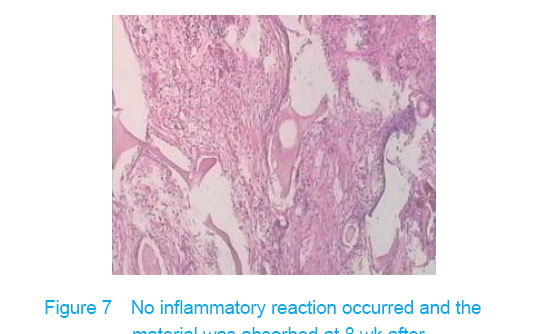
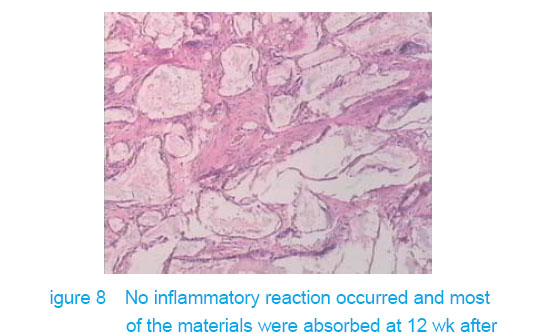
There was no wound infection, suppuration and rejection after subcutaneous implantation. Muscle color and texture was normal around material. At 4 weeks, there had slight inflammatory cell infiltration around the implantation and there had macrophage. Fibrous tissue grew to the interior of material and the fibers arranged disorderly. At 8 weeks, the morphology of muscle tissue was normal around materials, regular collagen fibers appeared and there was no imflammatory cell infiltration. At 12 weeks, part of the materials degraded and were replaced by newly developed fibrous tissue (Figures 6-8).
| [1]Liu BY, Ma XH, Li NY, et al. Biocompatibility and cytocompatibility of calcined Bone. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2008;12(41): 8055-8058.[2]Qiao GY, Su JS. Advances of composite scaffold materials for bone tissue engineering. Kouqiang Hemian Waike Zazhi. 2010;20(5):374-377.[3]Deligianni DD, Katsala ND, Koutsoukos PG, et al. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials. 2001;22(1):87-96.[4]Liu BY, Li NY, Fan GW, et al. Experimental study of cultivation, identification and induced differentiation of bone marrow stromal stem cells into osteoblasts in rats. Qilu Yixue Zazhi. 2007;22(3):215-217.[5]The Ministry of Science and Technology of the People′s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.[6]Liu BY, Li NY, Fan GW, et al. In vitro osteoblastic differentiation and identification of rat bone marrow mesenchymal stem cells by whole bone marrow adherent culture. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2007;11(50):10181-10184.[7]Zang HM, Liu YH, Chen JC, et al. An experimental research on different temperature sintered bone as carrier of bone morphogenetic protein. Shengwu Yixue Gongchengxue Zazhi. 2006;23(2):366-369.[8]Yang CL, Bi ZG, Wang YX, et al. The osteoblast differentiation of rabbit bone marrow stromal cells and construction with true bone ceramic. Zhongguo Guzhi Shusong Zazhi. 2005;11(3):329-332.[9]Lu W, Tao K, Mao TQ, et al. Experimental study of marrow stromal osteoMasts culture with caldned bone caldam. Zhongguo Linchuang Kangfu. 2003;7(2):214-215.[10]Zhang DZ, Fan QY, Ma BA, et al. Assessment on biological safety of material combined with true bone ceramic, bBMP and bone cement. Zhongguo Linchuang Kangfu. 2002;6(14):2060-2061.[11]Xu YH, Shi XY, Hu YY, et al. Osteogenetic activity in vivo of true bone ceramic with osteoblastic compound substances. Disi Junyi Daxue Xuebao. 2002;23(3):223-226.[12]Zheng QX, Liu SN. The preparation of sintered bovine cancellous bone and a study of its mechanical and chemical behavior and biocompatibility. Shengwu Yixue Gongchengxue Zazhi. 2005;22(1):95-98.[13]Li LF. Drug Experiment and Preparation. Beijing: University of Science and Technology of China Press. 2010:61,67,69.[14]Du Y, Ke ZY. Decalcified bone matrix and bone cement compound at various proportions in repairing rabbit femoral defect. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2009;13(21):4064-4068.[15]Wingerter S, Tucci M, Bumgardner J, et al. Evaluation of short-term healing following sustained delivery of osteoinductive agents in a rat femur drill defect model. Biomed Sci Instrum. 2007;43:188-193.[16]Cool SM, Kenny B, Wu A, et al. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)composite biomaterials for bone tissue regeneration: in vitro performance assessed by osteoblast proliferation, osteoclast adhesion and resorption, and macrophage proinflammatory response. J Biomed Mater Res A. 2007;82(3):599-610.[17]Leivo J, Meretoja V, Vippola M, et al. Sol-gel derived aluminosilicate coatings on alumina as substrate for osteoblasts. Acta Biomater. 2006;2(6):659-668.[18]Le Nihouannen D, Saffarzadeh A, Gauthier O, et al. Bone tissue formation in sheep muscles induced by a biphasic calcium phosphate ceramic and fibrin glue composite. J Mater Sci Mater Med. 2008;19(2):667-675.[19]Yan X, Huang X, Yu C, et al. The in-vitro bioactivity of mesoporous bioactive glasses. Biomaterials. 2006;27(18): 3396-3403.[20]Chen LQ, Li NY, Yuan RT, et al. Study of MSCs in vitro cultured on demineralized bone matrix of mongrel. Shanghai Kouqiang Yixue. 2007;16(3):255-258.[21]Li NY, Chen LQ, Chen T, et al. Effect of platelet-rich plasma and latissimus dorsi myofascia with blood vessel on vascularization of tissue engineered bone in dogs. Huaxi Kouqiang Yixue Zazhi. 2007;25(4):408-411. |
| [1] | 侯婧瑛, 于萌蕾, 郭天柱, 龙会宝, 吴 浩. 缺氧预处理激活HIF-1α/MALAT1/VEGFA通路促进骨髓间充质干细胞生存和血管再生[J]. 中国组织工程研究, 2021, 25(7): 985-990. |
| [2] | 梁学奇, 郭黎姣, 陈贺捷, 武 杰, 孙雅琪, 邢稚坤, 邹海亮, 陈雪玲, 吴向未. 泡状棘球绦虫原头蚴抑制骨髓间充质干细胞向成纤维细胞的分化[J]. 中国组织工程研究, 2021, 25(7): 996-1001. |
| [3] | 耿 瑶, 尹志良, 李兴平, 肖东琴, 侯伟光. hsa-miRNA-223-3p调控人骨髓间充质干细胞成骨分化的作用[J]. 中国组织工程研究, 2021, 25(7): 1008-1013. |
| [4] | 伦志刚, 金 晶, 王添艳, 李爱民. 过氧化物还原酶6干预骨髓间充质干细胞增殖及体外向神经谱系诱导分化[J]. 中国组织工程研究, 2021, 25(7): 1014-1018. |
| [5] | 朱雪芬, 黄 成, 丁 健, 戴永平, 刘元兵, 乐礼祥, 王亮亮, 杨建东. 胶质细胞神经营养因子诱导骨髓间充质干细胞向功能性神经元分化的机制[J]. 中国组织工程研究, 2021, 25(7): 1019-1025. |
| [6] | 裴丽丽, 孙贵才, 王 弟. 丹酚酸B抑制骨髓间充质干细胞氧化损伤及促进分化为心肌样细胞[J]. 中国组织工程研究, 2021, 25(7): 1032-1036. |
| [7] | 王诗琦, 张金生. 中医药调控缺血缺氧微环境对骨髓间充质干细胞增殖、分化及衰老的影响[J]. 中国组织工程研究, 2021, 25(7): 1129-1134. |
| [8] | 陈俊毅, 王 宁, 彭称飞, 朱伦井, 段江涛, 王 烨, 贝朝涌. 脱钙骨基质与慢病毒介导沉默P75神经营养因子受体转染骨髓间充质干细胞构建组织工程骨[J]. 中国组织工程研究, 2021, 25(4): 510-515. |
| [9] | 李 黎, 马 力. 磁性壳聚糖微球固定化乳糖酶及其酶学性质[J]. 中国组织工程研究, 2021, 25(4): 576-581. |
| [10] | 刘 旒, 周箐竹, 龚 桌, 刘博言, 杨 斌, 赵 娴. 胶原/无机材料构建组织工程骨的特点及制造技术[J]. 中国组织工程研究, 2021, 25(4): 607-613. |
| [11] | 姜 涛, 马 磊, 李志强, 寿 玺, 段明军, 吴 硕, 马 创, 魏 琴. 血小板衍生生长因子BB诱导骨髓间充质干细胞向血管内皮细胞分化[J]. 中国组织工程研究, 2021, 25(25): 3937-3942. |
| [12] | 刘利永, 周 雷. 组织工程用水凝胶研发现状和发展趋势:基于专利信息的分析[J]. 中国组织工程研究, 2021, 25(22): 3527-3533. |
| [13] | 何 林, 吴 稀, 何 淞, 杨 森. 经聚多巴胺涂层的羟基磷灰石生物陶瓷具有亲水性与细胞黏附性[J]. 中国组织工程研究, 2021, 25(22): 3540-3544. |
| [14] | 周安琪, 唐渝菲, 吴秉峰, 向 琳. 骨膜组织工程设计:共性与个性的结合[J]. 中国组织工程研究, 2021, 25(22): 3551-3557. |
| [15] | 郎丽敏, 何 生, 姜增誉, 胡奕奕, 张智星, 梁敏茜. 导电复合材料在心肌梗死组织工程治疗领域的应用进展[J]. 中国组织工程研究, 2021, 25(22): 3584-3590. |
A in vitro and in vivo comparative observation experiment.
The experiment was completed at Institute of Brain Science and Pharmacological Laboratory, Shanxi Datong University and Central Laboratory of Qingdao University from June 2011 to October 2011.
Animals
Wistar rats with (200±30) g of SPF grade, half male and half female, were purchased from Experimental Animal Center of Shanxi Medical University (animal certification number was SCXK(LU0)20030010). They were used for cell culture and the test of subcutaneous implantation; Kunming mice with (20±2) g, half male and half female, were provided by Animal Center of Shanxi Medical University. They were used for systemic acute toxicity test. During the experiment, animals treatment according with the Guidance Suggestions for the Care and Use of Laboratory Animals[5]. Fresh long bone epiphyseal end of adult bovine.
Sodium hydroxide analytically pure, hydrogen peroxide analytically pure, glycerine, anhydrous calcium chloride, anhydrous lithium chloride, chloroform, and methanol were purchased from Tianjin Rui-jinte industry chemical CO., LTD.
Box resistor furnace (DRZ-6 Tianjin Instrument Factory), scanning electron microscope (JEOL JSM -840, Japan), phase contrast microscope (OLYMPΜS, Japan), CO2 cells incubator (THERMOFORMA, America).
The third generation of bone marrow mesenchymal stem cells obtained with whole bone marrow culture method [6]
Male Wistar rats with 6 months old, weighing 180 g, were purchased from Experimental Animal Center of Shanxi Medical University. Animal certification number was SCXK (Jin) 2009-001. Bone marrow mesenchymal stem cells were primary and sub-cultured by their abilities to proliferate in culture with an attached well-spread morphology. The third generation of bone marrow mesenchymal stem cells were inoculated in the culture bottle and induced and differentiated. CD34 and CD45 were detected by flow cytometry. The morphological features of the cells were observed by saturated alizarin red staining and improved Kaplow method. The expression of osteocalcin, osteopontin and ONN mRNA were detected by reverse transcription-PCR.
Male adult Luxi Yellow cattle, 18 months old, weighing 350 kg, was purchased from market. The soft tissue and periosteum of long bone epiphyseal end of adult bovine was removed as possible and was cut off by diameter of about 2 cm×2 cm×1 cm, which washed by flowing water overnight, and then soaked in 0.5 mol/L solution of NaOH for 12 hours, degreased by placing in 1:1 chloroform and methanol extracts for 12 hours; soaked in 3% hydrogen peroxide for 24 hours, then to deproteinized by placing in 1:1:1 solution of 2 mol/L CaCl2, 0.5 mol/L ethylene diamine tetraacetic acid and 8 mol/L LiCl for 1 hour. Decalcified materials were washed three passes with the tri-distilled water, and then dried for 72 hours at 70 ℃. The bovine bones were put in porcelain bowl, and porcelain bowl was put in room temperature box type resistance furnace, and then connected the oxygen unit. Temperature increasing ratio of resistance furnace was 10 ℃/min, during this period oxygen was inlet continuously, paused for 30 minutes when temperature increased to 500 ℃ to avoid carbonization and bursting of bone, when temperature increased to 900 ℃,
lasted for 3 hours. Closed resistance furnace, natural cooled to room temperature, washed with distilled water, vacuum extracted and sterilized for spare use. Prepared true bone ceramic was soaked in Dulbecco’s modified eagle’s medium for 48 hours, the medium was changed for two times during this period, and the final pH value was 7.2-7.3. Materials were soaked in Dulbecco’s modified eagle’s medium containing 20% fetal bovine serum for 12 hours, at last adsorbed drying with sterile gauze for spare use.
True bone ceramic were dressed into cuboid with steel file for estimating volume (V=a×b×c), and then placed into graduated vessel which contained certain volume of glycerine beforehand. Thus the change of volume (V1) before and after can be obtained, porosity=
(1- V1/V)×100%, ten portions were measured and the average value was gained.
Fixed the materials with 2.5% glutaraldehyd for 24 hours, desiccated by ethanol, dried in vacuum, gold plated the surface, and then the ultrastructure was observed under scanning electron microscope.
The third generation of induced bone marrow mesenchymal stem cells were collected and then adjusted the cell concentration to 1×106/L, cell suspension was dropped on the true bone ceramic, made the cells entered into the deep part of scaffold until true bone ceramic completely soaked by cell suspension. Every true bone ceramic was added with
2 mL cell suspension and cultured in the incubator containing 5% CO2. The components and sources were 0.10 fetal bovine serum (Sijiqing Biology Technology Limited Company, Hangzhou)+low sugar Dulbecco’s modified eagle’s medium (Gibco)+100 U/L penicillin (SiGaole Kemao Limited Company, Shanghai)+100 U/L streptomycin (Xingyinhe Chemical Limited Company, Hubei)+2 mmol/L glutamine (Fu Runde Biology Technology Limited Company, Zhenzhou), 10 mmol/L β-sodium glycerophosphate (Sigma), 10-7 mol/L hexadecadrol (Sigma), 50 mg/L vitamin C (Sigma). The cell and scaffold morphology was observed with scanning electron microscope.
Hemolysis test [10]
10 mL fresh Chinchilla rabbit (male, 3 months, 2.5- 3.0 kg, provided by Animal Center of Shanxi Datong University) blood sample was taken through the ear vein with heparin as anticoagulant, equivalent volume of physiologic saline was added for spare use. And, 1 g material of true bone ceramic was grinded into shiver or powder, then put into 50 mL physiologic saline and soaked for 24 hours at 37 ℃. True bone ceramic water extract was sterilized by filtration with 0.2 μm of filter membrane. The experiment was divided into three groups: experimental group, negative control group and positive control group, each group had three tubes. In the experimental group, 10 mL water extract was added into 0.2 mL diluted blood. In the negative control group, 10 mL physiologic saline was added into 0.2 mL diluted blood. In the positive control group, 10 mL double distilled water was added into 0.2 mL diluted blood. The supernatant was carefully transferred after centrifuge (2 000 rpm, 5 minutes) in all tubes, and the centrifugal radius was 13.5 cm. The absorbance value was measured at UV-545 nm. The average value of three tests was obtained in every group. Hemolysis rate (%)=(absorbance value of experimental group-absorbance value of negative control group)/(absorbance value of positive control group-absorbance value of negative control group) ×100%. The evaluation standard: hemolysis extent > 5% was positive reaction, and this indicated that there have hemolytic phenomenon.
2 mL fresh blood plasma from Chinchilla rabbit (male, 3 months old, 2.5-3.0 kg, provided by Animal Center of Shanxi Datong University) and 100 mg powder of true bone ceramics were added, then the coagulation time was determined in the plasmozyme coagulation test by automatic coagulation radiometer. Partial thromboplastin cogaluation time, thromboplastin cogaluation time and the content of fibrin were measured, the influence of the material on blood coagulation function was detected.
A total of 20 Kunming mice, 4-6 weeks old, weighing 18-22 g, were purchased from Experimental Animal Center of Shanxi Medical University. Animal certification number was SCXK (Jin) 2009-001.The mice were randomly divided into two groups. All mice
of experimental group and control group were fasted for 12 hours, but water was given. 10 mL water extract (water extract was prepared with the method mentioned above) was injected into the tail vein in the experimental group, and 10 mL physiologic saline was injected into the tail vein in the control group. Mice were given free access to food and water, and the body mass, behavioral activity and death status for 7 consecutive days were observed. All mice were sacrificed at 7 days and autopsy was performed to observe the appearance shape and color of the heart, liver, spleen, lung and kidney. Death or adverse reaction was recorded. The tissue structure and cell morphology of the heart, liver and kidney were observed.
A total of 30 Kunming mice, 4-6 weeks old, weighing 18-22 g, were purchased from Experimental Animal Center of Shanxi Medical University. Animal certification number was SCXK (Jin) 2009-001, half male and half female, and the mice were randomly divided into three groups: 50 mL/kg water extract (water extract was prepared with the method mentioned above) was given by intraperitoneal injection in the experimental group, 50 mL/kg physiologic saline was injected in the negative control group, 100 mg/kg cyclophosphamide was injected in the positive control group. All mice were killed when the drug delivery had been terminated for 24 hours, and the femurs bone marrow smear was obtained for Giemsa staining. The numbers of bone marrow polychromative erythrocytes and micronucleus cells were counted and summed to 1 000 in each animal. The permillages of the micronucleus were calculated as well as the polychromatic erythrocytes/normal red blood cells ratios were explored at sampling time. Ratio > 1 or =1 is normal, and ratio < 1 may be the test substance produces toxicity to bone marrow cells.
A total of six Chinchilla rabbits (male, 3 months old, 2.0-3.0 kg, provided by Animal Center of Shanxi Datong University) were included, half male and half female. Hair on back was depilated, then the rabbits were observed for 2 days, and there was no bruise stimulation on the skin. Then, 0.1 mL water extract (water extract was prepared with the method mentioned above) and 0.1 mL physiologic saline was respectively injected at the two sides of the spine (ten points for each side). The injection site was observed for 7 days and scored according to Table 1.
A total of 3 albino rabbits (3 months old, 2.0-3.0 kg, provided by Animal Center of Shanxi Datong university) were included. The anus temperature of each rabbit was measured, once every 1 hour, twice as one observation course. The average value was used as normal body temperature. Then, 2.5 mL/kg water extract was injected via the ear vein after measuring body temperature for 15 minutes, once for 1 hour, three times were considered as one observation course. The rising temperature was the difference between highest body temperature and the normal body temperature. The judgment of fever reaction (the mean body temperature of rabbits was 38-39 ℃, and the difference value of highest and lowest temperature was less than 0.4 ℃, the difference value of normal body temperature in each rabbit should not exceed 1 ℃, if average increase of temperature> 0.6 ℃/rabbit or increase of the temperature of three rabbits was more than 1.4 ℃, which should be considered to be fever reaction).
A total of 12 Wistar rats, 8-9 months old, weighing 400-450 g, were purchased from Experimental Animal Center of Shanxi Medical University. Animal certification number was SCXK (Jin) 2009-001. They were anesthetized with intraperitoneal injection of 10% chloral hydrate (350 mg/kg). Two small incisions (1 cm) were made on the both sides of the skin and true bone ceramic (10 mm×10 mm×3 mm) was implanted. At last the incision was sutered. The animals were sacrificed at 4, 8 and 12 weeks after operation. Paraffin sections were prepared from the specimen. The degradation of materials, ultrastructure of true bone ceramic, cellular compatibility and biocompatibility were observed by hematoxylin-eosin staining.
Data were expressed as mean±SD for continuous variables. All statistical analyses were carried out using
SPSS 13.0 statistical software (SPSS, Chicago, IL, USA). A level of P < 0.05 was considered statistically significant.
True bone ceramic was eliminated antigenicity completely and had no immunological rejection after high-temperature sintering, which had good cell, tissue and blood compatibility and biological safety. It is convenience to get and acquire largely, process and store. It is easy to construct, thus its economic cost is far lower than the preparation of synthetic materials. It is one of the ideal tissue engineering scaffolds.
根据国际标准化组织公布的医疗器械生物学评价试验指南(ISO标准,1997)中基本评价的生物学实验,本文选择了溶血、凝血、刺激、致热、微核、急性全身毒性实验及皮下植入实验等几种较为常见的生物学方法评估煅烧骨的生物学安全性。
基金项目:
山西省高校科技研究开发项目(20111120)。
皮下植入实验是一种观察煅烧骨材料软组织相容性的有效方法。皮下植入实验显示煅烧骨植入Wistar大鼠后孔隙很快被纤维组织充满;4周时有轻微的炎症反应,是由手术创伤引起;8周时组织切片显示无炎症反应,纤维组织生长好,提示材料有良好的组织相容性;12周时个别区域出现轻度炎细胞浸润,多核巨细胞及异物吞噬反应仍较明显,推测与羟基磷灰石晶体的溶解引起细胞免疫反应有关。一般认为羟基磷灰石降解性能差,钙磷陶瓷在体内的生物吸收通常有两种方式,一是体液的侵蚀与溶解,其次为细胞的吞噬作用。钙磷陶瓷降解过程中巨噬细胞能够将细小的材料颗粒包裹并吞噬到细胞内形成吞噬体。实验发现12周时可见异物吞噬反应及多核巨细胞,这是由于羟基磷灰石晶体的溶解导致。从免疫学的角度来看,不同种属间植骨,其抗原差异巨大,移植后发生剧烈的免疫排斥反应,最终导致植骨失败。而煅烧骨经高温煅烧后,可以彻底消除其抗原性,不会产生免疫排斥反应,生物相容性好,因此具有很好的生物学安全性。
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||