中国组织工程研究 ›› 2018, Vol. 22 ›› Issue (29): 4721-4728.doi: 10.3969/j.issn.2095-4344.0632
• 干细胞综述 stem cell review • 上一篇 下一篇
间充质干细胞早衰的原因及预防
周 龙1,何 帆2,王 磊1
- 1南京医科大学附属苏州医院(苏州科技城医院)骨科,江苏省苏州市 215153;2苏州大学骨科研究所,江苏省苏州市 215007
-
修回日期:2018-07-16出版日期:2018-10-18发布日期:2018-10-18 -
通讯作者:王磊,副主任医师,南京医科大学附属苏州医院(苏州科技城医院)骨科,江苏省苏州市 215153 -
作者简介:周龙,男,1988年生,安徽省枞阳县人,汉族,2016年苏州大学毕业,硕士,医师,主要从事骨质疏松症的临床与相关基础研究。 -
基金资助:国家自然科学基金(31570978);国家自然科学基金青年科学基金(51203194)
Premature senescence of mesenchymal stem cells: causes and preventive measures
Zhou Long1, He Fan2, Wang Lei1
- 1Department of Orthopaedics, the Affiliated Suzhou Hospital of Nanjing Medical University (Suzhou Science and Technology Town Hospital), Suzhou 215153, Jiangsu Province, China; 2Orthopaedic Institute, Soochow University, Suzhou 215007, Jiangsu Province, China
-
Revised:2018-07-16Online:2018-10-18Published:2018-10-18 -
Contact:Wang Lei, Associate chief physician, Department of Orthopaedics, the Affiliated Suzhou Hospital of Nanjing Medical University (Suzhou Science and Technology Town Hospital), Suzhou 215153, Jiangsu Province, China -
About author:Zhou Long, Master, Physician, Department of Orthopaedics, the Affiliated Suzhou Hospital of Nanjing Medical University (Suzhou Science and Technology Town Hospital), Suzhou 215153, Jiangsu Province, China -
Supported by:the National Natural Science Foundation of China, No. 31570978; the National Natural Science Foundation of China for the Youth, No. 51203194
摘要:
文章快速阅读:
.jpg)
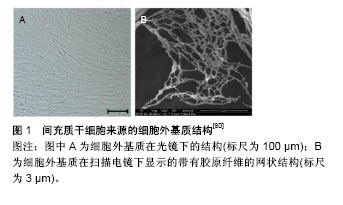
文题释义: 细胞衰老:是指DNA受到不可逆性损害,端粒缩短,细胞在生长过程中逐渐丧失自我更新能力,细胞周期停滞于DNA合成前期。间充质干细胞受到氧化应激刺激可提前进入衰老过程,即早衰。 预防间充质干细胞的衰老:间充质干细胞作为种子细胞可向成骨细胞、软骨细胞、脂肪细胞及神经细胞分化,预防及治疗其早衰为骨质疏松症、骨性关节炎等疾病的防治提供了一种新的思路。课题组先前的相关实验结果证实褪黑素及细胞外基质可显著提高干细胞的增殖及分化能力,有效预防骨髓间充质干细胞的早衰,在骨缺损方面有着重大的应用前景。
中图分类号:
引用本文
周 龙,何 帆,王 磊. 间充质干细胞早衰的原因及预防[J]. 中国组织工程研究, 2018, 22(29): 4721-4728.
Zhou Long, He Fan, Wang Lei. Premature senescence of mesenchymal stem cells: causes and preventive measures[J]. Chinese Journal of Tissue Engineering Research, 2018, 22(29): 4721-4728.
2.3.1 生长因子 生长因子可以维持并促进MSCs增殖及分化,但不能减缓端粒缩短的速率,增加端粒酶数量[60-61],见表1。5 μg/L成纤维细胞生长因子2可以在无论有无血清的培养环境下促进MSCs分裂增殖。Mizuta等[62]在兔膝关节损伤模型中发现成纤维细胞生长因子2可以通过促进MSCs增殖提升软骨细胞的修复能力。Ito等[13]也证实成纤维细胞生长因子2通过降低P21、P53、P16基因的表达水平,可以减缓人MSCs长期培养过程中出现的复制性衰老,也有可能是抑制转化生长因子β2 mRNA的表达来减少细胞停滞在G1期的比例。100 μg/L胰岛素生长因子1可通过调节转化生长因子β2促进老化MSCs产生蛋白多糖。

氧化应激引起细胞早衰,SIRT1通过调节与DNA损伤和修复有关的分子如FOXOs、P53、P21等够保护细胞免受早衰[101-103]。研究结果显示SIRT1通过调节FOXO3和衰老基因P53、P21的表达,避免氧化应激引起的肺细胞衰老[104]。Langley等[105]报道SIRT1的缺乏引起乙酰化P53的累积,这样增强了氧化应激诱导的衰老。在干细胞和祖细胞中,SIRT1也同样可以调节其衰老。组织蛋白酶可以减弱SIRT1在内皮祖细胞中的作用,导致氧化应激引起的早衰[106]。SIRT1参与维持干细胞体内平衡[107]。Homma等[108]揭示SIRT1在调节人胚胎干细胞和多能干细胞功能方面的重要作用。SIRT1也可以通过增强人端粒酶反转录基因的表达阻止人脐带MSCs的复制性衰老[109]。这些研究结果表明SIRT1对氧化应激环境下的干细胞复制性衰老和早衰调节的重要作用。
| [1] Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells.Science. 1999;284(5411):143-147. [2] Oreffo RO, Cooper C, Mason C, et al. Mesenchymal stem cells: lineage, plasticity, and skeletal therapeutic potential. Stem Cell Rev. 2005;1(2):169-178. [3] Vidal MA, Walker NJ, Napoli E, et al. Evaluation of senescence in mesenchymal stem cells isolated from equine bone marrow, adipose tissue, and umbilical cord tissue.Stem Cells Dev. 2012;21(2):273-283. [4] Brandl A, Meyer M, Bechmann V, et al. Oxidative stress induces senescence in human mesenchymal stem cells.Exp Cell Res. 2011;317(11):1541-1547. [5] Dumont P, Burton M, Chen QM, et al. Induction of replicative senescence biomarkers by sublethal oxidative stresses in normal human fibroblast.Free Radic Biol Med. 2000;28(3): 361-373. [6] Vacanti V, Kong E, Suzuki G, et al. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture.J Cell Physiol. 2005;205(2):194-201. [7] Mezey E.The therapeutic potential of bone marrow-derived stromal cells.J Cell Biochem. 2011;112(10):2683-2687. [8] He H, Liu X, Peng L, et al. Promotion of hepatic differentiation of bone marrow mesenchymal stem cells on decellularized cell-deposited extracellular matrix.Biomed Res Int. 2013; 2013:406871. [9] Kassem M, Marie PJ.Senescence-associated intrinsic mechanisms of osteoblast dysfunctions.Aging Cell. 2011; 10(2):191-197. [10] Li J, Pei M.Cell senescence: a challenge in cartilage engineering and regeneration.Tissue Eng Part B Rev. 2012; 18(4):270-287. [11] Campisi J, d'Adda di Fagagna F.Cellular senescence: when bad things happen to good cells.Nat Rev Mol Cell Biol. 2007; 8(9):729-740. [12] Krtolica A, Campisi J.Cancer and aging: a model for the cancer promoting effects of the aging stroma.Int J Biochem Cell Biol. 2002;34(11):1401-1414. [13] Ito T, Sawada R, Fujiwara Y, et al. FGF-2 suppresses cellular senescence of human mesenchymal stem cells by down-regulation of TGF-beta2.BiochemBiophys Res Commun. 2007;359(1):108-114. [14] Meza-Zepeda LA, Noer A, Dahl JA, et al. High-resolution analysis of genetic stability of human adipose tissue stem cells cultured to senescence.J Cell Mol Med. 2008;12(2):553-563. [15] Wagner W, Horn P, Castoldi M, et al. Replicative senescence of mesenchymal stem cells: a continuous and organized process.PLoS One. 2008;3(5):e2213. [16] Ryu E, Hong S, Kang J, et al. Identification of senescence-associated genes in human bone marrow mesenchymal stem cells.BiochemBiophys Res Commun. 2008;371(3):431-436. [17] Galderisi U, Helmbold H, Squillaro T, et al. In vitro senescence of rat mesenchymal stem cells is accompanied by downregulation of stemness-related and DNA damage repair genes.Stem Cells Dev. 2009;18(7):1033-1042. [18] Sawada R, Ito T, Tsuchiya T.Changes in expression of genes related to cell proliferation in human mesenchymal stem cells during in vitro culture in comparison with cancer cells.J Artif Organs. 2006;9(3):179-184. [19] Shelton DN, Chang E, Whittier PS, et al. Microarray analysis of replicative senescence.Curr Biol. 1999;9(17):939-945. [20] Zamore PD, Haley B.Ribo-gnome: the big world of small RNAs.Science. 2005;309(5740):1519-1524. [21] Fabbri M, Garzon R, Cimmino A, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B.Proc Natl AcadSci U S A. 2007;104(40):15805-15810. [22] Shibata KR, Aoyama T, Shima Y, et al. Expression of the p16INK4A gene is associated closely with senescence of human mesenchymal stem cells and is potentially silenced by DNA methylation during in vitro expansion.Stem Cells. 2007; 25(9):2371-2382. [23] Banfi A, Muraglia A, Dozin B, et al. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. ExpHematol. 2000;28(6):707-715. [24] Inoue K, Ohgushi H, Yoshikawa T, et al. The effect of aging on bone formation in porous hydroxyapatite: biochemical and histological analysis.J Bone Miner Res. 1997;12(6):989-994. [25] Digirolamo CM, Stokes D, Colter D, et al. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate.Br J Haematol. 1999;107(2):275-281. [26] Kozhevnikova MN, Mikaelian AS, Paiushina OV, et al. Comparative characterization of mesenchymal bone marrow stromal cells at early and late stages of culturing. IzvAkadNaukSer Biol. 2008;(2):156-162. [27] Mauney JR, Volloch V, Kaplan DL.Matrix-mediated retention of adipogenic differentiation potential by human adult bone marrow-derived mesenchymal stem cells during ex vivo expansion.Biomaterials. 2005;26(31):6167-6175. [28] Muraglia A, Cancedda R, Quarto R.Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model.J Cell Sci. 2000;113 ( Pt 7):1161-1166. [29] Park JS, Kim HY, Kim HW, et al. Increased caveolin-1, a cause for the declined adipogenic potential of senescent human mesenchymal stem cells.Mech Ageing Dev. 2005; 126(5):551-559. [30] Stewart SA, Ben-Porath I, Carey VJ, et al. Erosion of the telomeric single-strand overhang at replicative senescence. Nat Genet. 2003;33(4):492-496. [31] von Zglinicki T.Oxidative stress shortens telomeres.Trends Biochem Sci. 2002;27(7):339-344. [32] Kang MK, Guo W, Park NH.Replicative senescence of normal human oral keratinocytes is associated with the loss of telomerase activity without shortening of telomeres.Cell Growth Differ. 1998;9(1):85-95. [33] Kiyono T, Foster SA, Koop JI, et al. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells.Nature. 1998;396(6706): 84-88. [34] Ramirez RD, Morales CP, Herbert BS, et al. Putative telomere-independent mechanisms of replicative aging reflect inadequate growth conditions.Genes Dev. 2001;15(4): 398-403. [35] Graakjaer J, Christensen R, Kolvraa S, et al. Mesenchymal stem cells with high telomerase expression do not actively restore their chromosome arm specific telomere length pattern after exposure to ionizing radiation.BMC Mol Biol. 2007;8:49. [36] Guillot PV, Gotherstrom C, Chan J, et al. Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC.Stem Cells. 2007;25(3):646-654. [37] Baxter MA, Wynn RF, Jowitt SN, et al. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion.Stem Cells. 2004;22(5): 675-682. [38] Parsch D, Fellenberg J, Brümmendorf TH, et al. Telomere length and telomerase activity during expansion and differentiation of human mesenchymal stem cells and chondrocytes.J Mol Med (Berl). 2004;82(1):49-55. [39] Bonab MM, Alimoghaddam K, Talebian F, et al. Aging of mesenchymal stem cell in vitro.BMC Cell Biol. 2006;7:14. [40] Allsopp RC, Harley CB.Evidence for a critical telomere length in senescent human fibroblasts.Exp Cell Res. 1995;219(1): 130-136. [41] Bodnar AG, Ouellette M, Frolkis M, et al. Extension of life-span by introduction of telomerase into normal human cells.Science. 1998;279(5349):349-352. [42] Masutomi K, Yu EY, Khurts S, et al. Telomerase maintains telomere structure in normal human cells.Cell. 2003;114(2): 241-253. [43] Horikawa I, Barrett JC.Transcriptional regulation of the telomerase hTERT gene as a target for cellular and viral oncogenic mechanisms.Carcinogenesis. 2003;24(7): 1167-1176. [44] Bernardo ME, Zaffaroni N, Novara F, et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms.Cancer Res. 2007;67(19):9142-9149. [45] Serakinci N, Christensen R, Graakjaer J, et al. Ectopically hTERT expressing adult human mesenchymal stem cells are less radiosensitive than their telomerase negative counterpart.Exp Cell Res. 2007;313(5):1056-1067. [46] Kang SK, Putnam L, Dufour J, et al. Expression of telomerase extends the lifespan and enhances osteogenic differentiation of adipose tissue-derived stromal cells.Stem Cells. 2004; 22(7):1356-1372. [47] Shi S, Gronthos S, Chen S, et al. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression.Nat Biotechnol. 2002;20(6):587-591. [48] Simonsen JL, Rosada C, Serakinci N, et al. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotechnol. 2002;20(6):592-596. [49] Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts.Science. 1998; 282(5391):1145-1147. [50] Passos JF, Von Zglinicki T.Oxygen free radicals in cell senescence: are they signal transducers. Free Radic Res. 2006;40(12):1277-1283. [51] Allen RG, Tresini M, Keogh BP, et al. Differences in electron transport potential, antioxidant defenses, and oxidant generation in young and senescent fetal lung fibroblasts (WI-38).J Cell Physiol. 1999;180(1):114-122. [52] Passos JF, Saretzki G, Ahmed S, et al. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence.PLoS Biol. 2007;5(5):e110. [53] von Zglinicki T, Saretzki G, Döcke W, et al. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence. Exp Cell Res. 1995;220(1):186-193. [54] Chen QM, Prowse KR, Tu VC, et al. Uncoupling the senescent phenotype from telomere shortening in hydrogen peroxide-treated fibroblasts.Exp Cell Res. 2001;265(2): 294-303. [55] Choi KM, Seo YK, Yoon HH, et al. Effect of ascorbic acid on bone marrow-derived mesenchymal stem cell proliferation and differentiation.J BiosciBioeng. 2008;105(6):586-594. [56] Wang KH, Kao AP, Wangchen H, et al. Optimizing proliferation and characterization of multipotent stem cells from porcine adipose tissue.Biotechnol Appl Biochem. 2008; 51(Pt 4):159-166. [57] 李海生,陈金武,朱玲玲,等.持续低氧增强人骨髓间充质干细胞体外增殖[J].基础医学与临床,2005,25(3):268-271.[58] Ohnishi S, Yasuda T, Kitamura S, et al. Effect of hypoxia on gene expression of bone marrow-derived mesenchymal stem cells and mononuclear cells.Stem Cells. 2007;25(5): 1166-1177. [59] Lonergan T, Brenner C, Bavister B.Differentiation-related changes in mitochondrial properties as indicators of stem cell competence.J Cell Physiol. 2006;208(1):149-153. [60] Barbero A, Grogan S, Schäfer D, et al. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity.Osteoarthritis Cartilage. 2004;12(6):476-484. [61] Brandl A, Angele P, Roll C, et al. Influence of the growth factors PDGF-BB, TGF-beta1 and bFGF on the replicative aging of human articular chondrocytes during in vitro expansion.J Orthop Res. 2010;28(3):354-360. [62] Mizuta H, Kudo S, Nakamura E, et al. Active proliferation of mesenchymal cells prior to the chondrogenic repair response in rabbit full-thickness defects of articular cartilage.Osteoarthritis Cartilage. 2004;12(7):586-596. [63] Muscari C, Bonafe' F, Farruggia G, et al. Long-term treatment with N-acetylcysteine, but not caloric restriction, protects mesenchymal stem cells of aged rats against tumor necrosis factor-induced death.Exp Gerontol. 2006;41(8): 800-804. [64] Reiter RJ, Acuña-Castroviejo D, Tan DX, et al. Free radical-mediated molecular damage. Mechanisms for the protective actions of melatonin in the central nervous system.Ann N Y Acad Sci. 2001;939:200-215. [65] Tan DX, Manchester LC, Reiter RJ, et al. Identification of highly elevated levels of melatonin in bone marrow: its origin and significance.Biochim Biophys Acta. 1999;1472(1-2): 206-214. [66] Reiter RJ.Pineal melatonin: cell biology of its synthesis and of its physiological interactions.Endocr Rev. 1991;12(2): 151-180. [67] Galano A, Tan DX, Reiter RJ.On the free radical scavenging activities of melatonin's metabolites, AFMK and AMK.J Pineal Res. 2013;54(3):245-257. [68] Liu X, Gong Y, Xiong K, et al. Melatonin mediates protective effects on inflammatory response induced by interleukin-1 beta in human mesenchymal stem cells.J Pineal Res. 2013; 55(1):14-25. [69] Pei M, He F, Wei L, et al. Melatonin enhances cartilage matrix synthesis by porcine articular chondrocytes.J Pineal Res. 2009;46(2):181-187. [70] Hui JH, Li L, Teo YH, et al. Comparative study of the ability of mesenchymal stem cells derived from bone marrow, periosteum, and adipose tissue in treatment of partial growth arrest in rabbit.Tissue Eng. 2005;11(5-6):904-912. [71] Yu L, Sun Y, Cheng L, et al. Melatonin receptor-mediated protection against myocardial ischemia/reperfusion injury: role of SIRT1.J Pineal Res. 2014;57(2):228-238. [72] Proietti S, Cucina A, Dobrowolny G, et al. Melatonin down-regulates MDM2 gene expression and enhances p53 acetylation in MCF-7 cells.J Pineal Res. 2014;57(1):120-129. [73] Wang FW, Wang Z, Zhang YM, et al. Protective effect of melatonin on bone marrow mesenchymal stem cells against hydrogen peroxide-induced apoptosis in vitro.J Cell Biochem. 2013;114(10):2346-2355. [74] Gutierrez-Cuesta J, Tajes M, Jiménez A, et al. Evaluation of potential pro-survival pathways regulated by melatonin in a murine senescence model.J Pineal Res. 2008;45(4):497-505. [75] Zhou L, Chen X, Liu T, et al. Melatonin reverses H2O2 -induced premature senescence in mesenchymal stem cells via the SIRT1-dependent pathway. J Pineal Res. 2015;59(2): 190-205. [76] Kao CL, Chen LK, Chang YL, et al. Resveratrol protects human endothelium from H(2)O(2)-induced oxidative stress and senescence via SirT1 activation.J AtherosclerThromb. 2010;17(9):970-979. [77] Moussavi-Harami F, Duwayri Y, Martin JA, et al. Oxygen effects on senescence in chondrocytes and mesenchymal stem cells: consequences for tissue engineering.Iowa Orthop J. 2004;24:15-20. [78] Jin Y, Kato T, Furu M, et al. Mesenchymal stem cells cultured under hypoxia escape from senescence via down-regulation of p16 and extracellular signal regulated kinase. Biochem Biophys Res Commun. 2010;391(3):1471-1476. [79] Schrobback K, Klein TJ, Crawford R, et al. Effects of oxygen and culture system on in vitro propagation and redifferentiation of osteoarthritic human articular chondrocytes. Cell Tissue Res. 2012;347(3):649-663. [80] Liu X, Zhou L, Chen X, et al. Culturing on decellularized extracellular matrix enhances antioxidant properties of human umbilical cord-derived mesenchymal stem cells.Mater SciEng C Mater Biol Appl. 2016;61:437-448. [81] Badylak SF, Vorp DA, Spievack AR, et al. Esophageal reconstruction with ECM and muscle tissue in a dog model.J Surg Res. 2005;128(1):87-97. [82] Bhrany AD, Beckstead BL, Lang TC, et al. Development of an esophagus acellular matrix tissue scaffold.Tissue Eng. 2006; 12(2):319-330. [83] Brown B, Lindberg K, Reing J, et al. The basement membrane component of biologic scaffolds derived from extracellular matrix.Tissue Eng. 2006;12(3):519-526. [84] Ponce Márquez S, Martínez VS, McIntosh Ambrose W, et al. Decellularization of bovine corneas for tissue engineering applications.Acta Biomater. 2009;5(6):1839-1847. [85] Atala A, Vacanti JP, Peters CA, et al. Formation of urothelial structures in vivo from dissociated cells attached to biodegradable polymer scaffolds in vitro.J Urol. 1992;148(2 Pt 2):658-662. [86] Atala A, Freeman MR, Vacanti JP, et al. Implantation in vivo and retrieval of artificial structures consisting of rabbit and human urothelium and human bladder muscle.J Urol. 1993; 150(2 Pt 2):608-612. [87] Urist MR.The classic: a morphogenetic matrix for differentiation of bone tissue.Clin Orthop Relat Res. 2009; 467(12):3068-3070. [88] Linsley C, Wu B, Tawil B.The effect of fibrinogen, collagen type I, and fibronectin on mesenchymal stem cell growth and differentiation into osteoblasts.Tissue Eng Part A. 2013; 19(11-12):1416-1423. [89] Chen XD.Extracellular matrix provides an optimal niche for the maintenance and propagation of mesenchymal stem cells.Birth Defects Res C Embryo Today. 2010;90(1):45-54. [90] Cukierman E, Pankov R, Stevens DR, et al. Taking cell-matrix adhesions to the third dimension.Science. 2001;294(5547): 1708-1712. [91] Pei M, He F, Kish VL.Expansion on extracellular matrix deposited by human bone marrow stromal cells facilitates stem cell proliferation and tissue-specific lineage potential.Tissue Eng Part A. 2011;17(23-24):3067-3076. [92] Choi HR, Cho KA, Kang HT, et al. Restoration of senescent human diploid fibroblasts by modulation of the extracellular matrix.Aging Cell. 2011;10(1):148-157. [93] He F, Liu X, Xiong K, et al. Extracellular matrix modulates the biological effects of melatonin in mesenchymal stem cells.J Endocrinol. 2014;223(2):167-180. [94] Stein GH, Beeson M, Gordon L.Failure to phosphorylate the retinoblastoma gene product in senescent human fibroblasts. Science. 1990;249(4969):666-669. [95] Wang W, Chen JX, Liao R, et al. Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence.Mol Cell Biol. 2002;22(10):3389-3403. [96] Gray-Schopfer VC, Cheong SC, Chong H, et al. Cellular senescence in naevi and immortalisation in melanoma: a role for p16. Br J Cancer. 2006;95(4):496-505. [97] Beauséjour CM, Krtolica A, Galimi F, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22(16):4212-4222. [98] Zhang DY, Wang HJ, Tan YZ.Wnt/β-catenin signaling induces the aging of mesenchymal stem cells through the DNA damage response and the p53/p21 pathway.PLoS One. 2011; 6(6):e21397. [99] Dai SM, Shan ZZ, Nakamura H, et al. Catabolic stress induces features of chondrocyte senescence through overexpression of caveolin 1: possible involvement of caveolin 1-induced down-regulation of articular chondrocytes in the pathogenesis of osteoarthritis.Arthritis Rheum. 2006; 54(3):818-831. [100] Kregel KC, Zhang HJ.An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations.Am J Physiol RegulIntegr Comp Physiol. 2007; 292(1):R18-36. [101] Yao H, Rahman I.Perspectives on translational and therapeutic aspects of SIRT1 in inflammaging and senescence. Biochem Pharmacol. 2012;84(10):1332-1339. [102] Furukawa A, Tada-Oikawa S, Kawanishi S, et al. H2O2 accelerates cellular senescence by accumulation of acetylated p53 via decrease in the function of SIRT1 by NAD+ depletion. Cell Physiol Biochem. 2007;20(1-4):45-54. [103] Tsuji T, Aoshiba K, Nagai A.Cigarette smoke induces senescence in alveolar epithelial cells.Am J Respir Cell Mol Biol. 2004;31(6):643-649. [104] Yao H, Chung S, Hwang JW, et al. SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice.J Clin Invest. 2012;122(6):2032-2045. [105] Langley E, Pearson M, Faretta M, et al. Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence.EMBO J. 2002;21(10):2383-2396. [106] Chen J, Xavier S, Moskowitz-Kassai E, et al. Cathepsin cleavage of sirtuin 1 in endothelial progenitor cells mediates stress-induced premature senescence.Am J Pathol. 2012; 180(3):973-983. [107] Yuan HF, Zhai C, Yan XL, et al. SIRT1 is required for long-term growth of human mesenchymal stem cells.J Mol Med (Berl). 2012;90(4):389-400. [108] Homma K, Sone M, Taura D, et al. Sirt1 plays an important role in mediating greater functionality of human ES/iPS-derived vascular endothelial cells.Atherosclerosis. 2010;212(1):42-47. [109] Yamashita S, Ogawa K, Ikei T, et al. SIRT1 prevents replicative senescence of normal human umbilical cord fibroblast through potentiating the transcription of human telomerase reverse transcriptase gene.BiochemBiophys Res Commun. 2012;417(1):630-634. |
| [1] | 蒲 锐, 陈子扬, 袁凌燕. 不同细胞来源外泌体保护心脏的特点与效应[J]. 中国组织工程研究, 2021, 25(在线): 1-. |
| [2] | 林清凡, 解一新, 陈婉清, 叶振忠, 陈幼芳. 人胎盘源间充质干细胞条件培养液可上调缺氧状态下BeWo细胞活力和紧密连接因子的表达[J]. 中国组织工程研究, 2021, 25(在线): 4970-4975. |
| [3] | 张秀梅, 翟运开, 赵 杰, 赵 萌. 类器官模型国内外数据库近10年文献研究热点分析[J]. 中国组织工程研究, 2021, 25(8): 1249-1255. |
| [4] | 王正东, 黄 娜, 陈婧娴, 郑作兵, 胡鑫宇, 李 梅, 苏 晓, 苏学森, 颜 南. 丁酸钠抑制氟中毒可诱导小胶质细胞活化及炎症因子表达增多[J]. 中国组织工程研究, 2021, 25(7): 1075-1080. |
| [5] | 汪显耀, 关亚琳, 刘忠山. 提高间充质干细胞治疗难愈性创面的策略[J]. 中国组织工程研究, 2021, 25(7): 1081-1087. |
| [6] | 万 然, 史 旭, 刘京松, 王岩松. 间充质干细胞分泌组治疗脊髓损伤的研究进展[J]. 中国组织工程研究, 2021, 25(7): 1088-1095. |
| [7] | 廖成成, 安家兴, 谭张雪, 王 倩, 刘建国. 口腔鳞状细胞癌干细胞的治疗靶点及应用前景[J]. 中国组织工程研究, 2021, 25(7): 1096-1103. |
| [8] | 谢文佳, 夏天娇, 周卿云, 刘羽佳, 顾小萍. 小胶质细胞介导神经元损伤在神经退行性疾病中的作用[J]. 中国组织工程研究, 2021, 25(7): 1109-1115. |
| [9] | 李珊珊, 郭笑霄, 尤 冉, 杨秀芬, 赵 露, 陈 曦, 王艳玲. 感光细胞替代治疗视网膜变性疾病[J]. 中国组织工程研究, 2021, 25(7): 1116-1121. |
| [10] | 焦 慧, 张一宁, 宋雨晴, 林 宇, 王秀丽. 乳腺癌类器官研究进展及临床应用前景[J]. 中国组织工程研究, 2021, 25(7): 1122-1128. |
| [11] | 王诗琦, 张金生. 中医药调控缺血缺氧微环境对骨髓间充质干细胞增殖、分化及衰老的影响[J]. 中国组织工程研究, 2021, 25(7): 1129-1134. |
| [12] | 曾燕华, 郝延磊. 许旺细胞体外培养及纯化的系统性综述[J]. 中国组织工程研究, 2021, 25(7): 1135-1141. |
| [13] | 孔德胜, 何晶晶, 冯宝峰, 郭瑞云, Asiamah Ernest Amponsah, 吕 飞, 张舒涵, 张晓琳, 马 隽, 崔慧先. 间充质干细胞修复大动物模型脊髓损伤疗效评价的Meta分析[J]. 中国组织工程研究, 2021, 25(7): 1142-1148. |
| [14] | 侯婧瑛, 于萌蕾, 郭天柱, 龙会宝, 吴 浩. 缺氧预处理激活HIF-1α/MALAT1/VEGFA通路促进骨髓间充质干细胞生存和血管再生[J]. 中国组织工程研究, 2021, 25(7): 985-990. |
| [15] | 史洋洋, 秦英飞, 吴福玲, 何 潇, 张雪静. 胎盘间充质干细胞预处理预防小鼠毛细支气管炎[J]. 中国组织工程研究, 2021, 25(7): 991-995. |
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
1.3 资料提取与文献质量评价 通过阅读标题和摘要进行筛选,排除中英文文献重复报道和与纳入标准无关的文献。查阅全文,进一步判断与纳入标准一致的文章,最终保留109篇文献做深入分析。以此为依据对MSCs早衰的原因及其预防措施进行归纳和总结并进行展望。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
.jpg)
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||