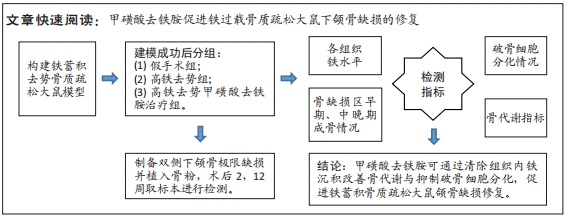
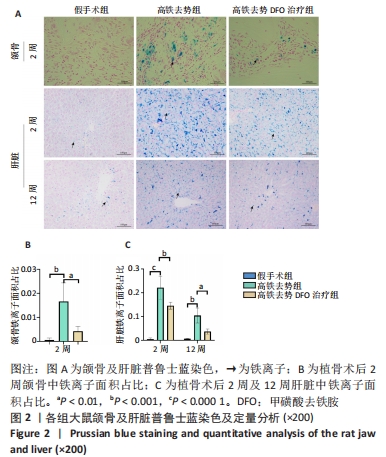
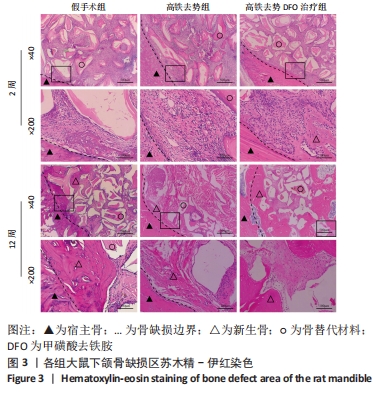
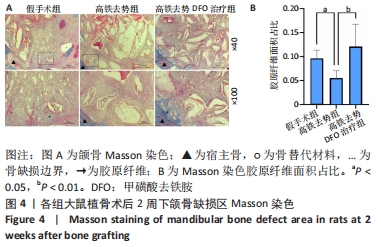
[1] Management of Osteoporosis in Postmenopausal Women: The 2021 Position Statement of The North American Menopause Society’’ Editorial Panel. Management of osteoporosis in postmenopausal women: the 2021 position statement of The North American Menopause Society. Menopause. 2021;28(9):973-997.
[2] TAO ZS, LI TL, WEI S. Silymarin prevents iron overload induced bone loss by inhibiting oxidative stress in an ovariectomized animal model. Chem Biol Interact. 2022;366:110168.
[3] LU M, LIU Y, SHAO M, et al. Associations of Iron Intake, Serum Iron and Serum Ferritin with Bone Mineral Density in Women: The National Health and Nutrition Examination Survey, 2005-2010. Calcif Tissue Int. 2020;106(3): 232-238.
[4] BAJBOUJ K, SHAFARIN J, ALLAM H, et al. Elevated Levels of Estrogen Suppress Hepcidin Synthesis and Enhance Serum Iron Availability in Premenopausal Women. Exp Clin Endocrinol Diabetes. 2018;126(7):453-459.
[5] HUANG X, XU Y, PARTRIDGE NC. Dancing with sex hormones, could iron contribute to the gender difference in osteoporosis? Bone. 2013;55(2):458-460.
[6] ZIMMERMANN MB, HURRELL RF. Nutritional iron deficiency. Lancet. 2007; 370(9586):511-520.
[7] LI GF, PAN YZ, SIROIS P, et al. Iron homeostasis in osteoporosis and its clinical implications. Osteoporos Int. 2012;23(10):2403-2408.
[8] XUE Y, YANG J, LUO J, et al. Disorder of Iron Metabolism Inhibits the Recovery of Unloading-Induced Bone Loss in Hypomagnetic Field. J Bone Miner Res. 2020;35(6):1163-1173.
[9] GAUDIO A, MORABITO N, CATALANO A, et al. Pathogenesis of Thalassemia Major-associated Osteoporosis: A Review with Insights from Clinical Experience. J Clin Res Pediatr Endocrinol. 2019;11(2):110-117.
[10] XU S, ZHANG G, GUO JF, et al. Associations between osteoporosis and risk of periodontitis: A pooled analysis of observational studies. Oral Dis. 2021;27(2):357-369.
[11] HONG SJ, YANG BE, YOO DM, et al. Analysis of the relationship between periodontitis and osteoporosis/fractures: a cross-sectional study. BMC Oral Health. 2021;21(1):125.
[12] DREYER H, GRISCHKE J, TIEDE C, et al. Epidemiology and risk factors of peri-implantitis: A systematic review. J Periodontal Res. 2018;53(5):657-681.
[13] LIU LN, ZHANG XH, LIU HH, et al. Osteogenesis Differences Around Titanium Implant and in Bone Defect Between Jaw Bones and Long Bones. J Craniofac Surg. 2020;31(8):2193-2198.
[14] OLEJNIK C, FALGAYRAC G, DURING A, et al. Doses effects of zoledronic acid on mineral apatite and collagen quality of newly-formed bone in the rat’s calvaria defect. Bone. 2016;89:32-39.
[15] LIN W, LI Q, ZHANG D, et al. Mapping the immune microenvironment for mandibular alveolar bone homeostasis at single-cell resolution. Bone Res. 2021;9(1):17.
[16] CHEN B, YAN YL, LIU C, et al. Therapeutic effect of deferoxamine on iron overload-induced inhibition of osteogenesis in a zebrafish model. Calcif Tissue Int. 2014;94(3):353-360.
[17] 陈爱华,赵国阳,庄琴,等.去铁胺对输血相关性铁过载患者骨密度的影响[J].中国骨质疏松杂志,2018,24(3):311-314.
[18] 郑妮娜,卢俊玲,盛慧.甲磺酸去铁胺辅助治疗绝经后骨质疏松症的可行性和安全性研究[J].中国骨质疏松杂志,2019,25(7):998-1001.
[19] LIU Y, LIU J, CAI F, et al. Hypoxia During the Consolidation Phase of Distraction Osteogenesis Promotes Bone Regeneration. Front Physiol. 2022;13:804469.
[20] 杜文瑜,杨静文,姜婷.甲磺酸去铁胺促进大鼠颅骨临界骨缺损血管化骨再生的早期连续观察[J].北京大学学报(医学版),2021,53(6):1171-1177.
[21] HOLDEN P, NAIR LS. Deferoxamine: An Angiogenic and Antioxidant Molecule for Tissue Regeneration. Tissue Eng Part B Rev. 2019;25(6):461-470.
[22] LANE JM, RUSSELL L, KHAN SN. Osteoporosis. Clin Orthop Relat Res. 2000; (372):139-150.
[23] GUIGLIA R, DI FEDE O, LO RUSSO L, et al. Osteoporosis, jawbones and periodontal disease. Med Oral Patol Oral Cir Bucal. 2013;18(1):e93-99.
[24] WANG B, FENG C, LIU Y, et al. Recent advances in biofunctional guided bone regeneration materials for repairing defective alveolar and maxillofacial bone: A review. Jpn Dent Sci Rev. 2022;58:233-248.
[25] MARDAS N, BUSETTI J, DE FIGUEIREDO JA, et al. Guided bone regeneration in osteoporotic conditions following treatment with zoledronic acid. Clin Oral Implants Res. 2017;28(3):362-371.
[26] YOUSEFZADEH N, KASHFI K, JEDDI S, et al. Ovariectomized rat model of osteoporosis: a practical guide. EXCLI J. 2020;19:89-107.
[27] WANG Z, DUAN R, JIN Y, et al. Effects of ferric ammonium citrate on iron accumulation, bone turnover and bone density in ovariectomized rat models with osteoporosis. Cell Mol Biol (Noisy-le-grand). 2022;68(8):163-166.
[28] ZHANG C, MENG T, DAN Z, et al. Minocycline ameliorates osteoporosis induced by ovariectomy (OVX) and iron accumulation via iron chelation, bone metabolism regulation and inhibition of oxidative stress. QJM. 2020: hcaa271. doi: 10.1093/qjmed/hcaa271.
[29] DÍAZ-CASTRO J, LÓPEZ-FRÍAS MR, CAMPOS MS, et al. Severe nutritional iron-deficiency anaemia has a negative effect on some bone turnover biomarkers in rats. Eur J Nutr. 2012;51(2):241-247.
[30] TAO H, GE G, LIANG X, et al. ROS signaling cascades: dual regulations for osteoclast and osteoblast. Acta Biochim Biophys Sin (Shanghai). 2020; 52(10):1055-1062.
[31] BALOGH E, PARAGH G, JENEY V. Influence of Iron on Bone Homeostasis. Pharmaceuticals (Basel). 2018;11(4):107.
[32] ZANDI M, DEHGHAN A, GHEYSARI F, et al. Evaluation of teriparatide effect on healing of autografted mandibular defects in rats. J Craniomaxillofac Surg. 2019;47(1):120-126.
[33] ZHAO J, HU J, WANG S, et al. Combination of beta-TCP and BMP-2 gene-modified bMSCs to heal critical size mandibular defects in rats. Oral Dis. 2010;16(1):46-54.
[34] 高超.甲磺酸去铁胺对去势小鼠、大鼠骨质疏松模型影响的实验研究[D].苏州:苏州大学,2013.
[35] 刘江锋.低氧模拟剂对膝关节置换术后钛假体骨整合影响和术前骨质疏松、骨代谢指标及危险因素的相关研究[D].石家庄:河北医科大学,2022.
[36] ZHANG J, HU W, DING C, et al. Deferoxamine inhibits iron-uptake stimulated osteoclast differentiation by suppressing electron transport chain and MAPKs signaling. Toxicol Lett. 2019;313:50-59.
[37] ZHANG J, ZHAO H, YAO G, et al. Therapeutic potential of iron chelators on osteoporosis and their cellular mechanisms. Biomed Pharmacother. 2021;137:111380.
[38] JIANG Z, WANG H, QI G, et al. Iron overload-induced ferroptosis of osteoblasts inhibits osteogenesis and promotes osteoporosis: An in vitro and in vivo study. IUBMB Life. 2022;74(11):1052-1069.
[39] JIA P, CHEN H, KANG H, et al. Deferoxamine released from poly(lactic-co-glycolic acid) promotes healing of osteoporotic bone defect via enhanced angiogenesis and osteogenesis. J Biomed Mater Res A. 2016;104(10): 2515-2527.
[40] 苏州大学. 甲磺酸去铁胺在制备治疗绝经后骨质疏松疾病药物中的用途:CN201110437117.2[P]. 2012-07-04.
|