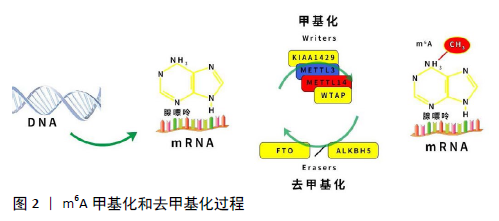
[1] DUBIN DT, TAYLOR RH. The methylation state of poly A-containing messenger RNA from cultured hamster cells. Nucleic Acids Res. 1975; 2(10):1653-1668.
[2] 张翔, 杜娟, 陈雅慧, 等. mRNA m6A甲基化修饰异常与疾病的研究进展[J]. 生命的化学,2019,39(2):255-261.
[3] DESROSIERS R, FRIDERICI K, ROTTMAN F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974;71(10):3971-3975.
[4] HOROWITZ S, HOROWITZ A, NILSEN TW, et al. Mapping of N6-methyladenosine residues in bovine prolactin mRNA. Proc Natl Acad Sci U S A. 1984;81(18):5667-5671.
[5] KRUG RM, MORGAN MA, SHATKIN AJ. Influenza viral mRNA contains internal N6-methyladenosine and 5’-terminal 7-methylguanosine in cap structures. J Virol. 1976;20(1):45-53.
[6] TRAUBE FR, CARELL T. The chemistries and consequences of DNA and RNA methylation and demethylation. RNA Biol. 2017;14(9):1099-1107.
[7] HUANG H, WENG H, CHEN J. The Biogenesis and Precise Control of RNA m(6)A Methylation. Trends Genet. 2020;36(1):44-52.
[8] WEI CM, GERSHOWITZ A, MOSS B. Methylated nucleotides block 5’ terminus of HeLa cell messenger RNA. Cell. 1975;4(4):379-386.
[9] DOMINISSINI D, MOSHITCH-MOSHKOVITZ S, SCHWARTZ S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485(7397):201-206.
[10] CAO G, LI H B, YIN Z, et al. Recent advances in dynamic m6A RNA modification. Open Biol. 2016;6(4):160003.
[11] MEYER KD, SALETORE Y, ZUMBO P, et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell. 2012;149(7):1635-1646.
[12] LI M, ZHAO X, WANG W, et al. Ythdf2-mediated m(6)A mRNA clearance modulates neural development in mice. Genome Biol. 2018;19(1):69.
[13] ZHANG C, CHEN Y, SUN B, et al. m(6)A modulates haematopoietic stem and progenitor cell specification. Nature. 2017;549(7671):273-276.
[14] WANG X, LU Z, GOMEZ A, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505(7481): 117-120.
[15] ALDERMAN MR, XIAO AZ. N(6)-Methyladenine in eukaryotes. Cell Mol Life Sci. 2019;76(15):2957-2966.
[16] LENCE T, PAOLANTONI C, WORPENBERG L, et al. Mechanistic insights into m(6)A RNA enzymes. Biochim Biophys Acta Gene Regul Mech. 2019;1862(3):222-229.
[17] LIU J, YUE Y, HAN D, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10(2):93-95.
[18] PING XL, SUN BF, WANG L, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24(2):177-189.
[19] LIAO S, SUN H, XU C. YTH Domain: A Family of N(6)-methyladenosine (m(6)A) Readers. Genomics Proteomics Bioinformatics. 2018;16(2): 99-107.
[20] ZHAO W, QI X, LIU L, et al. Epigenetic Regulation of m(6)A Modifications in Human Cancer. Mol Ther Nucleic Acids. 2020;19:405-412.
[21] YUE Y, LIU J, HE C. RNA N6-methyladenosine methylation in post- transcriptional gene expression regulation. Genes Dev. 2015;29(13): 1343-1355.
[22] LIU ZX, LI LM, SUN HL, et al. Link Between m6A Modification and Cancers. Front Bioeng Biotechnol. 2018;6:89.
[23] LIU N, DAI Q, ZHENG G, et al. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518(7540):560-564.
[24] ANDERSEN TL, SONDERGAARD TE, SKORZYNSKA KE, et al. A physical mechanism for coupling bone resorption and formation in adult human bone. Am J Pathol. 2009;174(1):239-247.
[25] KIM M, KIM C, CHOI YS, et al. Age-related alterations in mesenchymal stem cells related to shift in differentiation from osteogenic to adipogenic potential: implication to age-associated bone diseases and defects. Mech Ageing Dev. 2012;133(5):215-225.
[26] CAMPI G, CRISTOFARO F, PANI G, et al. Heterogeneous and self-organizing mineralization of bone matrix promoted by hydroxyapatite nanoparticles. Nanoscale. 2017;9(44):17274-17283.
[27] BATEMAN ME, STRONG AL, MCLACHLAN JA, et al. The Effects of Endocrine Disruptors on Adipogenesis and Osteogenesis in Mesenchymal Stem Cells: A Review. Front Endocrinol (Lausanne). 2016;7:171.
[28] NAKASHIMA K, DE CROMBRUGGHE B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet. 2003; 19(8):458-466.
[29] DELGADO-CALLE J, SAÑUDO C, SÁNCHEZ-VERDE L, et al. Epigenetic regulation of alkaline phosphatase in human cells of the osteoblastic lineage. Bone. 2011;49(4):830-838.
[30] YE L, FAN Z, YU B, et al. Histone Demethylases KDM4B and KDM6B Promote Osteogenic Differentiation of Human MSCs. Cell Stem Cell. 2018;23(6):898-899.
[31] TIAN C, HUANG Y, LI Q, et al. Mettl3 Regulates Osteogenic Differentiation and Alternative Splicing of Vegfa in Bone Marrow Mesenchymal Stem Cells. Int J Mol Sci. 2019;20(3):551.
[32] YAN G, YUAN Y, HE M, et al. m(6)A Methylation of Precursor-miR-320/RUNX2 Controls Osteogenic Potential of Bone Marrow-Derived Mesenchymal Stem Cells. Mol Ther Nucleic Acids. 2020;19:421-436.
[33] LI D, CAI L, MENG R, et al. METTL3 Modulates Osteoclast Differentiation and Function by Controlling RNA Stability and Nuclear Export. Int J Mol Sci. 2020;21(5):1660.
[34] YAO Y, BI Z, WU R, et al. METTL3 inhibits BMSC adipogenic differentiation by targeting the JAK1/STAT5/C/EBPβ pathway via an m(6)A-YTHDF2-dependent manner. FASEB J. 2019;33(6):7529-7544.
[35] CARMELIET P, NG YS, NUYENS D, et al. Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nat Med. 1999;5(5):495-502.
[36] QIAO H, ZHANG R, GAO L, et al. Molecular Imaging for Comparison of Different Growth Factors on Bone Marrow-Derived Mesenchymal Stromal Cells’ Survival and Proliferation In Vivo. Biomed Res Int. 2016; 2016:1363902.
[37] ZHANG J, GUAN J, QI X, et al. Dimethyloxaloylglycine Promotes the Angiogenic Activity of Mesenchymal Stem Cells Derived from iPSCs via Activation of the PI3K/Akt Pathway for Bone Regeneration. Int J Biol Sci. 2016;12(6):639-652.
[38] ZHANG J, LIU X, LI H, et al. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res Ther. 2016;7(1):136.
[39] WU Y, XIE L, WANG M, et al. Mettl3-mediated m(6)A RNA methylation regulates the fate of bone marrow mesenchymal stem cells and osteoporosis. Nat Commun. 2018;9(1):4772.
[40] JIN M, SONG S, GUO L, et al. Increased serum GDF11 concentration is associated with a high prevalence of osteoporosis in elderly native Chinese women. Clin Exp Pharmacol Physiol. 2016;43(11):1145-1147.
[41] LU Q, TU ML, LI CJ, et al. GDF11 Inhibits Bone Formation by Activating Smad2/3 in Bone Marrow Mesenchymal Stem Cells. Calcif Tissue Int. 2016;99(5):500-509.
[42] LIU W, ZHOU L, ZHOU C, et al. GDF11 decreases bone mass by stimulating osteoclastogenesis and inhibiting osteoblast differentiation. Nat Commun. 2016;7:12794.
[43] SHEN GS, ZHOU HB, ZHANG H, et al. The GDF11-FTO-PPARγ axis controls the shift of osteoporotic MSC fate to adipocyte and inhibits bone formation during osteoporosis. Biochim Biophys Acta Mol Basis Dis. 2018;1864(12):3644-3654.
[44] DIEPPE PA, LOHMANDER LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005;365(9463):965-973.
[45] HWANG HS, KIM HA. Chondrocyte Apoptosis in the Pathogenesis of Osteoarthritis. Int J Mol Sci. 2015;16(11):26035-26054.
[46] LIU Q, LI M, JIANG L, et al. METTL3 promotes experimental osteoarthritis development by regulating inflammatory response and apoptosis in chondrocyte. Biochem Biophys Res Commun. 2019; 516(1):22-27.
[47] ZHANG Y, GU X, LI D, et al. METTL3 Regulates Osteoblast Differentiation and Inflammatory Response via Smad Signaling and MAPK Signaling. Int J Mol Sci. 2019;21(1):199.
[48] KASSEM A, HENNING P, LUNDBERG P, et al. Porphyromonas gingivalis Stimulates Bone Resorption by Enhancing RANKL (Receptor Activator of NF-κB Ligand) through Activation of Toll-like Receptor 2 in Osteoblasts. J Biol Chem. 2015;290(33):20147-20158.
[49] SAINT-PASTOU TC, GASQUE P. Bone responses in health and infectious diseases: A focus on osteoblasts. J Infect. 2017;75(4):281-292.
[50] ZHANG Y, XU J, RUAN YC, et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat Med. 2016;22(10):1160-1169.
[51] SON JH, CHO YC, SUNG IY, et al. Melatonin promotes osteoblast differentiation and mineralization of MC3T3-E1 cells under hypoxic conditions through activation of PKD/p38 pathways. J Pineal Res. 2014;57(4):385-392.
[52] LI D, LIU J, GUO B, et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat Commun. 2016;7:10872.
[53] 薛徽, 孙瑶. 影响骨折愈合的生物因素研究新进展[J]. 口腔医学, 2018,38(11):1043-1047.
[54] MI B, XIONG Y, YAN C, et al. Methyltransferase-like 3-mediated N6-methyladenosine modification of miR-7212-5p drives osteoblast differentiation and fracture healing. J Cell Mol Med. 2020;24(11): 6385-6396.
[55] VIJAYAMURUGAN N, BAKHSHI S. Review of management issues in relapsed osteosarcoma. Expert Rev Anticancer Ther. 2014;14(2):151-161.
[56] HUANG Y, SU R, SHENG Y, et al. Small-Molecule Targeting of Oncogenic FTO Demethylase in Acute Myeloid Leukemia. Cancer Cell. 2019;35(4):677-691.
[57] ZHANG C, SAMANTA D, LU H, et al. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m⁶A-demethylation of NANOG mRNA. Proc Natl Acad Sci U S A. 2016; 113(14):E2047-E2056.
[58] BARBIERI I, TZELEPIS K, PANDOLFINI L, et al. Promoter-bound METTL3 maintains myeloid leukaemia by m(6)A-dependent translation control. Nature. 2017;552(7683):126-131.
[59] MIAO W, CHEN J, JIA L, et al. The m6A methyltransferase METTL3 promotes osteosarcoma progression by regulating the m6A level of LEF1. Biochem Biophys Res Commun. 2019;516(3):719-725.
[60] ZHOU L, YANG C, ZHANG N, et al. Silencing METTL3 inhibits the proliferation and invasion of osteosarcoma by regulating ATAD2. Biomed Pharmacother. 2020;125:109964.
[61] LI J, RAO B, YANG J, et al. Dysregulated m6A-Related Regulators Are Associated With Tumor Metastasis and Poor Prognosis in Osteosarcoma. Front Oncol. 2020;10:769.
|