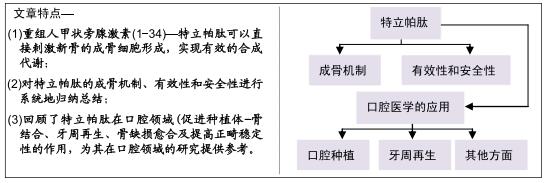
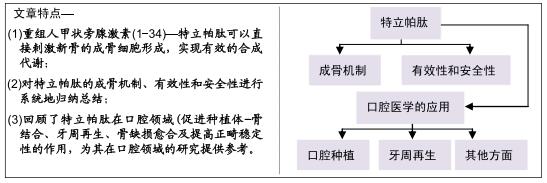
|
[1] 冯钰,刘福祥.骨质疏松症对颌骨的影响及其治疗的研究进展[J].国际口腔医学杂志,2008,35(S1):11-13.
[2] KANIS JA, COOPER C, RIZZOLI R, et al. Executive summary of the European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Calcif Tissue Int. 2019; 104(3):235-238.
[3] POLYZOS SA, MAKRAS P, EFSTATHIADOU Z, et al. Investigational parathyroid hormone receptor analogs for the treatment of osteoporosis. Expert Opin Investig Drugs. 2015; 24(2):145-157.
[4] BARON R, HESSE E. Update on bone anabolics in osteoporosis treatment: rationale, current status, and perspectives. J Clin Endocrinol Metab. 2012;97(2):311-325.
[5] BOGADO CE, MASSARI FE, ZANCHETTA JR. Parathyroid hormone (1-84) and teriparatide in the treatment of postmenopausal osteoporosis. Womens Health (Lond). 2006; 2(3):447-457.
[6] KEUTMANN HT, SAUER MM, HENDY GN, et al. Complete amino acid sequence of human parathyroid hormone. Biochemistry. 1978;17(26):5723-5729.
[7] LANGDAHL BL, ANDERSEN JD. Treatment of Osteoporosis: Unmet Needs and Emerging Solutions. J Bone Metab. 2018;25(3): 133-140.
[8] 王敏娇,司家文,沈国芳.促进骨形成的甲状旁腺激素:作用及机制[J].中国组织工程研究,2015,19(15):2405-2409.
[9] 陈茜,曹国颖.新型骨质疏松症治疗药abaloparatide的作用机制与临床评价[J].临床药物治疗杂志,2018,16(7):37-40.
[10] BELLIDO T, ALI AA, PLOTKIN LI, et al. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. J Biol Chem. 2003;278(50):50259-50272.
[11] QIN L, LI X, KO JK, et al. Parathyroid hormone uses multiple mechanisms to arrest the cell cycle progression of osteoblastic cells from G1 to S phase. J Biol Chem. 2005;280(4):3104-3111.
[12] JILKA RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40(6):1434-1446.
[13] WINKLER DG, SUTHERLAND MK, GEOGHEGAN JC, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22(23):6267-6276.
[14] KHOSLA S, WESTENDORF JJ, OURSLER MJ. Building bone to reverse osteoporosis and repair fractures. J Clin Invest. 2008; 118(2):421-428.
[15] BIKLE DD, SAKATA T, LEARY C, et al. Insulin-like growth factor I is required for the anabolic actions of parathyroid hormone on mouse bone. J Bone Miner Res. 2002;17(9):1570-1578.
[16] FOX J. Developments in parathyroid hormone and related peptides as bone-formation agents. Curr Opin Pharmacol. 2002;2(3):338-344.
[17] NEER RM, ARNAUD CD, ZANCHETTA JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434-1441.
[18] YOO JI, HA YC, RYU HJ, et al. Teriparatide Treatment in Elderly Patients With Sacral Insufficiency Fracture. J Clin Endocrinol Metab. 2017;102(2):560-565.
[19] KITAGUCHI K, KASHII M, EBINA K, et al. Effects of Weekly Teriparatide Administration for Vertebral Stability and Bony Union in Patients with Acute Osteoporotic Vertebral Fractures. Asian Spine J. 2019;13(5):763-771.
[20] CHEN Q, GUO M, MA X, et al. Adherence to Teriparatide Treatment and Risk of Fracture: A Systematic Review and Meta-Analysis. Horm Metab Res. 2019;51(12):785-791.
[21] 徐陆晨,李运峰.骨质疏松性骨折药物治疗的研究进展[J].中国骨质疏松杂志,2017,23(7):947-953.
[22] ANDREWS EB, GILSENAN A, MIDKIFF K, et al. Challenges in studying very rare cancer outcomes and infrequent exposures: example of teriparatide and osteosarcoma. Ann Epidemiol. 2016;26(11):751-753.
[23] DRAKE MT, SRINIVASAN B, MÖDDER UI, et al. Effects of intermittent parathyroid hormone treatment on osteoprogenitor cells in postmenopausal women. Bone. 2011;49(3):349-355.
[24] GREY A. Teriparatide for bone loss in the jaw. N Engl J Med. 2010;363(25):2458-2459.
[25] PAZIANAS M. Anabolic effects of PTH and the 'anabolic window'. Trends Endocrinol Metab. 2015;26(3):111-113.
[26] NAKAMURA T, SUGIMOTO T, NAKANO T, et al. Randomized Teriparatide [human parathyroid hormone (PTH) 1-34] Once-Weekly Efficacy Research (TOWER) trial for examining the reduction in new vertebral fractures in subjects with primary osteoporosis and high fracture risk. J Clin Endocrinol Metab. 2012;97(9):3097-3106.
[27] USUI T, FUNAGOSHI M, SETO K, et al. Persistence of and switches from teriparatide treatment among women and men with osteoporosis in the real world: a claims database analysis. Arch Osteoporos. 2018;13(1):54.
[28] KUMAGAI Y, OSE A, TANAKA K, et al. Safety Profiles, Pharmacokinetics, and Changes in Bone Turnover Markers After Twice-Weekly Subcutaneous Administration of Teriparatide in Healthy Japanese Postmenopausal Women: A Single-Blind Randomized Study. Clin Pharmacol Drug Dev. 2020;9(1):87-96.
[29] COSMAN F, LANE NE, BOLOGNESE MA, et al. Effect of transdermal teriparatide administration on bone mineral density in postmenopausal women. J Clin Endocrinol Metab. 2010;95(1): 151-158.
[30] HÄMMERLE SP, MINDEHOLM L, LAUNONEN A, et al. The single dose pharmacokinetic profile of a novel oral human parathyroid hormone formulation in healthy postmenopausal women. Bone. 2012;50(4):965-973.
[31] PEARSON RG, MASUD T, BLACKSHAW E, et al. Nasal Administration and Plasma Pharmacokinetics of Parathyroid Hormone Peptide PTH 1-34 for the Treatment of Osteoporosis. Pharmaceutics. 2019;11(6): E265.
[32] GUAÑABENS N, MORO-ÁLVAREZ MJ, CASADO E, et al. The next step after anti-osteoporotic drug discontinuation: an up-to-date review of sequential treatment. Endocrine. 2019;64(3): 441-455.
[33] VOHRA F, AL-RIFAIY MQ, ALMAS K, et al. Efficacy of systemic bisphosphonate delivery on osseointegration of implants under osteoporotic conditions: lessons from animal studies. Arch Oral Biol. 2014;59(9):912-920.
[34] BEPPU K, KIDO H, WATAZU A, et al. Peri-implant bone density in senile osteoporosis-changes from implant placement to osseointegration. Clin Implant Dent Relat Res. 2013;15(2): 217-226.
[35] DARCEY J, HORNER K, WALSH T, et al. Tooth loss and osteoporosis: to assess the association between osteoporosis status and tooth number. Br Dent J. 2013;214(4):E10.
[36] ANIL S, PREETHANATH RS, ALMOHARIB HS, et al. Impact of osteoporosis and its treatment on oral health. Am J Med Sci. 2013;346(5):396-401.
[37] SHIROTA T, TASHIRO M, OHNO K, et al. Effect of intermittent parathyroid hormone (1-34) treatment on the bone response after placement of titanium implants into the tibia of ovariectomized rats. J Oral Maxillofac Surg. 2003;61(4):471-480.
[38] ALMAGRO MI, ROMAN-BLAS JA, BELLIDO M, et al. PTH [1-34] enhances bone response around titanium implants in a rabbit model of osteoporosis. Clin Oral Implants Res. 2013;24(9): 1027-1034.
[39] JIANG L, ZHANG W, WEI L, et al. Early effects of parathyroid hormone on vascularized bone regeneration and implant osseointegration in aged rats. Biomaterials. 2018;179:15-28.
[40] KUCHLER U, LUVIZUTO ER, TANGL S, et al. Short-term teriparatide delivery and osseointegration: a clinical feasibility study. J Dent Res. 2011;90(8):1001-1006.
[41] KRAUS D, JÄGER A, ABUDUWALI N, et al. Intermittent PTH(1-34) signals through protein kinase A to regulate osteoprotegerin production in human periodontal ligament cells in vitro. Clin Oral Investig. 2012;16(2):611-618.
[42] AUSENDA F, RASPERINI G, ACUNZO R, et al. New Perspectives in the Use of Biomaterials for Periodontal Regeneration. Materials (Basel). 2019;12(13): E2197.
[43] VASCONCELOS DF, MARQUES MR, BENATTI BB, et al. Intermittent parathyroid hormone administration improves periodontal healing in rats. J Periodontol. 2014;85(5):721-728.
[44] SILVA MA, VASCONCELOS DF, MARQUES MR, et al. Parathyroid hormone intermittent administration promotes delay on rat incisor eruption. Arch Oral Biol. 2016;69:102-108.
[45] DE OLIVEIRA PUTTINI I, GOMES-FERREIRA PHDS, DE OLIVEIRA D, et al. Teriparatide improves alveolar bone modelling after tooth extraction in orchiectomized rats. Arch Oral Biol. 2019; 102:147-154.
[46] BASHUTSKI JD, EBER RM, KINNEY JS, et al. Teriparatide and osseous regeneration in the oral cavity. N Engl J Med. 2010; 363(25):2396-2405.
[47] LI F, LI G, HU H, et al. Effect of parathyroid hormone on experimental tooth movement in rats. Am J Orthod Dentofacial Orthop. 2013;144(4):523-532.
[48] LOSSDÖRFER S, YILDIZ F, GÖTZ W, et al. Anabolic effect of intermittent PTH(1-34) on the local microenvironment during the late phase of periodontal repair in a rat model of tooth root resorption. Clin Oral Investig. 2010;14(1):89-98.
[49] ZANDI M, DEHGHAN A, GHEYSARI F, et al. Evaluation of teriparatide effect on healing of autografted mandibular defects in rats. J Craniomaxillofac Surg. 2019;47(1):120-126.
[50] ZANDI M, DEHGHAN A, AMINI P, et al. Evaluation of the effect of teriparatide therapy on mandibular fracture healing in rats with medication-related osteonecrosis of the jaw. Clin Oral Investig. 2019;23(11):3987-3993.
[51] KAKEHASHI H, ANDO T, MINAMIZATO T, et al. Administration of teriparatide improves the symptoms of advanced bisphosphonate-related osteonecrosis of the jaw: preliminary findings. Int J Oral Maxillofac Surg. 2015;44(12):1558-1564.
[52] KIM JY, PARK JH, JUNG HD, et al. Treatment of Medication-Related Osteonecrosis of the Jaw Around the Dental Implant With a Once-Weekly Teriparatide: A Case Report and Literature Review. J Oral Implantol. 2019;45(5):403-407.
[53] JUNG J, YOO HY, KIM GT, et al. Short-Term Teriparatide and Recombinant Human Bone Morphogenetic Protein-2 for Regenerative Approach to Medication-Related Osteonecrosis of the Jaw: A Preliminary Study. J Bone Miner Res. 2017;32(12): 2445-2452.
[54] CHA YH, HONG N, RHEE Y, et al. Teriparatide therapy for severe, refractory osteoradionecrosis of the jaw. Osteoporos Int. 2018; 29(4):987-992.
[55] VALDERRAMA P, JUNG RE, THOMA DS, et al. Evaluation of parathyroid hormone bound to a synthetic matrix for guided bone regeneration around dental implants: a histomorphometric study in dogs. J Periodontol. 2010;81(5):737-747.
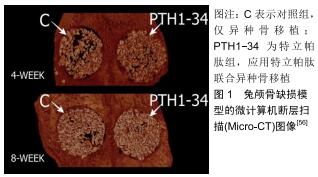
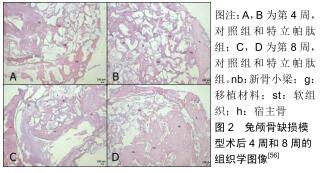
[56]ÖZER T, BAŞLARLI Ö, AKTAŞ A, et al. Locally administrated single-dose teriparatide affects critical-size rabbit calvarial defects: A histological, histomorphometric and micro-CT study. Acta Orthop Traumatol Turc. 2019;53(6):478-484.
|