| [1] Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166:189-197.[2] Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13(3):269-288.[3] Chen SC, Brown PR, Rosie DM. Extraction procedures for use prior to HPLC nucleotide analysis using microparticle chemically bonded packings. J Chromatogr Sci. 1977;15(6):218-221.[4] Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967-978.[5] Johnstone RM, Adam M, Hammond JR, et al. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262:9412-9420.[6] Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19(2):43-51.[7] Sahoo S, Losordo DW. Exosomes and Cardiac Repair After Myocardial Infarction. Circ Res. 2014;114:333-344.[8] Yu F, Jun A,Yusuke Y, et al. Suppression of autophagy by extracellular vesicles promotes myofibroblast differentiation in COPD pathogenesis. J Extracell Vesicles. 2015;4:28388.[9] Yang Y, Li YY. Exosomal transfer of miR-30a between cardiomyocytes regulates autophagy after hypoxia. J Mol Med .2016;94(6):711-724.[10] Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195-208.[11] Lener T, Gimona M, Aigner L, et al. Applying extracellular vesicles based therapeutics in clinical trials an ISEV position paper. J Extracell Vesicles. 2015;4:30087.[12] Wu YT, Deng WT. Exosomes: improved methods to characterize their morphology,RNA content, and surface protein biomarkers. Analyst. 2015;140(19):6631-6642.[13] Yin M, Loyer X, Boulanger CM. Extracellular vesicles as new pharmacological targets to treat atherosclerosis. Eur J Pharmacol. 2015;763(Pt A):90-103.[14] Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373-383.[15] Pugholm LH, Revenfeld AL, Søndergaard EK, et al. Antibody-Based Assays for Phenotyping of Extracellular Vesicles. Biomed Res Int. 2015;2015:524817.[16] Kalra H, Simpson RJ, Ji H, et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10(12):e1001450.[17] Chen JY, An R, Liu ZJ, et al. Therapeutic effects of mesenchymal stem cell-derived microvesicles on pulmonary arterial hypertension in rats. Acta Pharmacologica Sinica. 2014;35(9):1121-1128.[18] Witwer KW, Buzás EI, Bemis LT, et al.Standardization of sample collection,isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2:20360. [19] Chen JY, Liu ZJ, Hong MM, et al. Proangiogenic Compositions of Microvesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells. PLoS One. 2014;9(12):e115316.[20] Cai J, Han Y, Ren HM, et al. Extracellular vesicle-mediated transfer of donor genomic DNA to recipient cells is a novel mechanism for genetic influence between cells. J Mol Cell Biol. 2013;5(4):227-238.[21] Lasda E, Parker R. Circular RNAs Co-Precipitate with Extracellular Vesicles: a Possible Mechanism for circRNA Clearance. PLoS One. 2016;11(2):e0148407. [22] Yuana Y, Sturk A, Nieuwland R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013; 27:31-39.[23] Sunkara V, Woo HK, Cho YK. Emerging techniques in the isolation and characterization of extracellular vesicles and their roles in cancer diagnostics and prognostics. Analyst. 2015.[24] Iraci N, Leonardi T, Gessler F, et al. Focus on extracellular vesicles: physiological role and signalling properties of extracellular membrane vesicles. Int J Mol Sci. 2016;17(2):171. [25] Ratajczak J, Miekus K, Kucia M, et al. Embryonic stem cellderived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20(5):847-856.[26] Takahashi K, Yan IK. Involvement of extracellular vesicle long noncoding RNA (linc-VLDLR) in tumor cell responses to chemotherapy. Mol Cancer Res. 2014;12(10):1377-1387. [27] Collino F, Deregibus MC, Bruno S, et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010;5(7):e11803.[28] Mause SF, Weber C. Microparticles Protagonists of a Novel Communication Network for Intercellular Information Exchange. Circ Res. 2010;107(9):1047-1057.[29] Li L, Piontek K, Ishida M, et al. Extracellular vesicles carry miR-195 to intrahepatic cholangiocarcinoma andimprove survival in a rat model.Hepatology. 2016.[30] Helbing T, Olivier C, Bode C, et al. Role of microparticles in endothelial dysfunction and arterial hypertension. World J Cardiol. 2014;6(11):1135-1139.[31] Zakharova L, Svetlova M, Fomina AF. T cell exosomes induce cholesterol accumulation in human monocytes via phosphatidylserine receptor. J Cell Physiol. 2007;212:174-181.[32] Chaput N, Théry C. Exosomes: immune properties and potential clinical implementations. Semin Immunopathol. 2011; 33:419-440.[33] Montoro-García S, Shantsila E, Tapp LD, et al. Small-size circulating microparticles in acute coronary syndromes: relevance to fibrinolytic status, reparative markers and outcomes. Atherosclerosis. 2013;227(2):313-322.[34] Lorenzen JM, Thum T. Circulating and urinary microRNAs in kidney disease. Clin J Am Soc Nephrol. 2012;7(9):1528-1533.[35] Fleitas T, Martínez-Sales V, Vila V, et al. Circulating endothelial cells and microparticles as prognostic markers in advanced non-small cell lung cancer. PLoS One. 2012;7(10):e47365.[36] Melo SA, Luecke LB, Kahlert C, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523(7559):177-182.[37] Bank IE, Timmers L, Gijsberts CM, et al. The diagnostic and prognostic potential of plasma extracellular vesicles for cardiovascular disease. Exp Rev Mol Diagn. 2015;15(12): 1577-1588. [38] Wisgrill l, Lamm C. Peripheral blood microvesicles secretion is influenced by storage time, temperature, and anticoagulants. Cytometry A. 2016;89(7):663-672.[39] Ong SG, Wu JC. Exosomes as potential alternatives to stem cell therapy in mediating cardiac regeneration. Circ Res. 2015; 117:7-9. [40] Ong SG, Lee WH. Cross talk of combined gene and cell therapy in ischemic heart disease role of exosomal microrna transfer. circulation. 2014;130(suppl 1):S60-S69.[41] Lai RC, Yeo RW, Tan KH, et al. Exosomes for drug delivery a novel application for the mesenchymal stem cell. Biotechnol Adv. 2013;31:543-551.[42] Yim N, Ryu SW. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat Commun. 2016;7:12277.[43] Ohno S, Takanashi M, Sudo K, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21(1):185-191.[44] Ibrahim AG, Cheng K, Marbán E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports. 2014;2(5):606-619. |
.jpg) 文题释义:
细胞外囊泡:是由脂质双分子层包绕形成的球状膜性囊泡,是细胞自发或在一定条件下释放出的一种亚细胞成份,实质上是一组纳米级颗粒,近年来学界研究的热点,包括外泌体、膜微粒及微囊泡等。
自噬:这是发生在细胞代谢过程以清除代谢废物或维持细胞器更新的一种现象,其过程如下:首先细胞的一些成分将部分细胞质和(或)细胞内需降解的细胞器、蛋白质等成分包裹起来形成自噬体,接着自噬体与溶酶体融合形成自噬溶酶体,降解其所包裹的内容物,目前研究发现自噬在机体的生理和病理过程中均能见到。
文题释义:
细胞外囊泡:是由脂质双分子层包绕形成的球状膜性囊泡,是细胞自发或在一定条件下释放出的一种亚细胞成份,实质上是一组纳米级颗粒,近年来学界研究的热点,包括外泌体、膜微粒及微囊泡等。
自噬:这是发生在细胞代谢过程以清除代谢废物或维持细胞器更新的一种现象,其过程如下:首先细胞的一些成分将部分细胞质和(或)细胞内需降解的细胞器、蛋白质等成分包裹起来形成自噬体,接着自噬体与溶酶体融合形成自噬溶酶体,降解其所包裹的内容物,目前研究发现自噬在机体的生理和病理过程中均能见到。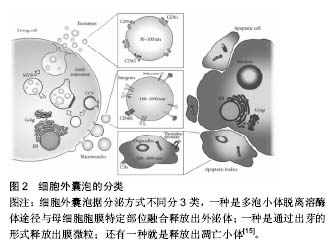
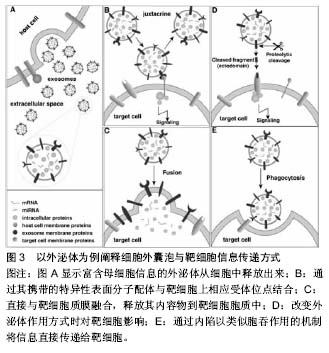
.jpg)
.jpg) 文题释义:
细胞外囊泡:是由脂质双分子层包绕形成的球状膜性囊泡,是细胞自发或在一定条件下释放出的一种亚细胞成份,实质上是一组纳米级颗粒,近年来学界研究的热点,包括外泌体、膜微粒及微囊泡等。
自噬:这是发生在细胞代谢过程以清除代谢废物或维持细胞器更新的一种现象,其过程如下:首先细胞的一些成分将部分细胞质和(或)细胞内需降解的细胞器、蛋白质等成分包裹起来形成自噬体,接着自噬体与溶酶体融合形成自噬溶酶体,降解其所包裹的内容物,目前研究发现自噬在机体的生理和病理过程中均能见到。
文题释义:
细胞外囊泡:是由脂质双分子层包绕形成的球状膜性囊泡,是细胞自发或在一定条件下释放出的一种亚细胞成份,实质上是一组纳米级颗粒,近年来学界研究的热点,包括外泌体、膜微粒及微囊泡等。
自噬:这是发生在细胞代谢过程以清除代谢废物或维持细胞器更新的一种现象,其过程如下:首先细胞的一些成分将部分细胞质和(或)细胞内需降解的细胞器、蛋白质等成分包裹起来形成自噬体,接着自噬体与溶酶体融合形成自噬溶酶体,降解其所包裹的内容物,目前研究发现自噬在机体的生理和病理过程中均能见到。