中国组织工程研究 ›› 2015, Vol. 19 ›› Issue (34): 5473-5479.doi: 10.3969/j.issn.2095-4344.2015.34.013
• 材料生物相容性 material biocompatibility • 上一篇 下一篇
新型含铜钛合金的生物相容性
任宝瑞1,刘 杰2,张二林3,董 会4
- 1佳木斯大学附属口腔医院,黑龙江省佳木斯市 154007; 2青岛大学附属医学院,山东省青岛市 266000; 3东北大学,辽宁省沈阳市 110819;4齐齐哈尔医学院附属第三医院,黑龙江省齐齐哈尔市 161000
Biocompatibility of a new titanium alloy containing copper
Ren Bao-rui1, Liu Jie2, Zhang Er-lin3, Dong Hui4
- 1Stomatological Hospital of Jiamusi University, Jiamusi 154007, Heilongjiang Province, China; 2Medical School of Qingdao University, Qingdao 266000, Shandong Province, China; 3Northeastern University, Shenyang 110819, Liaoning Province, China; 4Second Affiliated Hospital of Qiqihar Medical University, Qiqihar 161000, Heilongjiang Province, China
摘要:
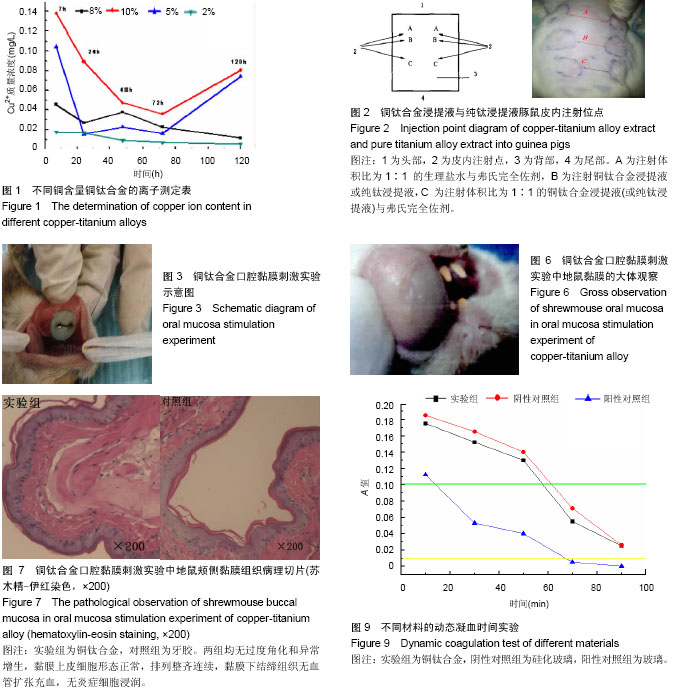
背景:为提高钛材料的抗菌性及生物相容性,前期研究制备了铜钛合金材料。
目的:参照GB/T16886-ISO10993所规定的原则和实验方法对铜钛合金进行较为全面、系统的生物相容性评价。
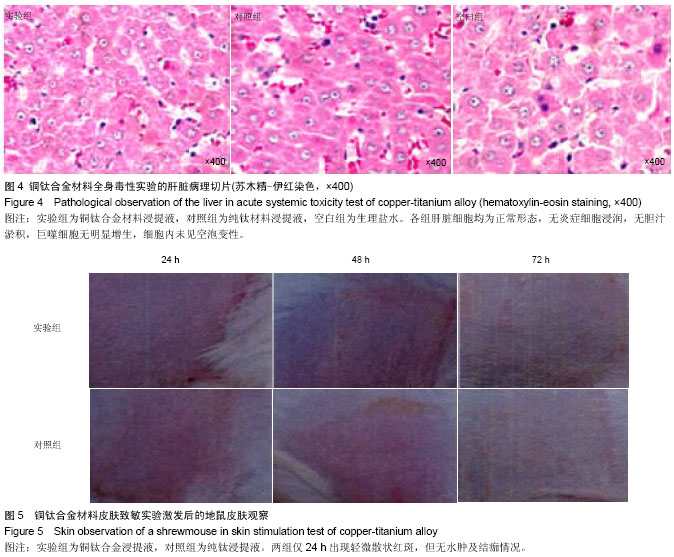
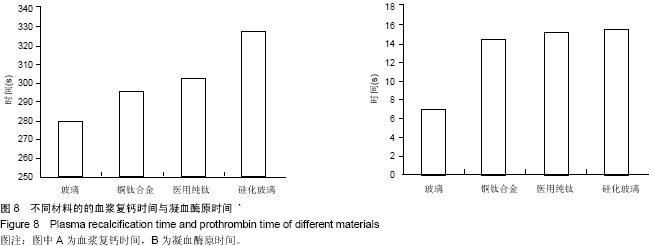
方法:根据预实验结果,选择含铜量10%的铜钛合金材料,通过口腔黏膜刺激实验、皮肤刺激实验、短期全身毒性实验、溶血实验、血浆复钙时间实验、凝血酶原时间实验及动态凝血实验验证铜钛合金的生物相容性。
结果与结论:铜钛合金材料无口腔黏膜刺激性、无短期全身毒性、无溶血性、无皮肤致敏反应,具有良好的血液相容性。
中图分类号: