中国组织工程研究 ›› 2020, Vol. 24 ›› Issue (13): 1996-2004.doi: 10.3969/j.issn.2095-4344.2048
• 脂肪干细胞 adipose-derived stem cells • 上一篇 下一篇
人脂肪干细胞来源外泌体对四氯化碳诱导肝纤维化模型大鼠的治疗作用
李洪超1,王 皙1,李 莉2,李震宇3,臧祖盛3,周 桁3,王晓今3,陈成伟3,程明亮1,吴 君1,金银鹏2,傅青春2
- 1贵州医科大学附属医院,贵州省贵阳市 550004;2复旦大学附属上海公共卫生临床中心,上海市 201508;3中国人民解放军海军905医院上海市肝病研究中心,上海市 200235
-
收稿日期:2019-03-13修回日期:2019-03-18接受日期:2019-04-30出版日期:2020-05-08发布日期:2020-03-07 -
通讯作者:傅青春,硕士,主任医师,复旦大学附属上海公共卫生临床中心,上海市 201508 吴君,硕士,主任医师,贵州医科大学附属医院,贵州省贵阳市 550004 金银鹏,硕士,主治医师,复旦大学附属上海公共卫生临床中心,上海市 201508 -
作者简介:李洪超,男,1991年生,山东省菏泽市人,汉族,2018年贵州医科大学毕业,硕士,主要从事干细胞研究。 并列第一作者:王皙,女,1993年生,贵州省毕节地区人,汉族,2019年贵州医科大学毕业,硕士,主要从事干细胞研究。 -
基金资助:南京军区医学创新重大课题(14ZX01);中国肝炎防治基金-天晴肝病研究基金项目(TQGB20150104)
Therapeutic effect of human adipose stem cells derived exosomes on carbon tetrachloride induced liver fibrosis in rats
Li Hongchao1, Wang Xi1, Li Li2, Li Zhenyu3, Zang Zusheng3, Zhou Heng3, Wang Xiaojin3, Chen Chengwei3, Cheng Mingliang1, Wu Jun1, #br# Jin Yinpeng2, Fu Qingchun2
- 1Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China; 2Shanghai Public Health Clinical Center, Fudan University, Shanghai 201508, China; 3Shanghai Liver Diseases Research Center, the 905 Hospital of PLA Navy, Shanghai 200235, China
-
Received:2019-03-13Revised:2019-03-18Accepted:2019-04-30Online:2020-05-08Published:2020-03-07 -
Contact:Fu Qingchun, Master, Chief physician, Shanghai Public Health Clinical Center, Fudan University, Shanghai 201508, China Wu Jun, Master, Chief physician, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China Jin Yinpeng, Master, Attending physician, Shanghai Public Health Clinical Center, Fudan University, Shanghai 201508, China -
About author:Li Hongchao, Master, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China Wang Xi, Master, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China Both Li Hongchao and Wang Xi contributed equally to this paper. -
Supported by:Nanjing Military Region Medical Innovation Major Project, No. 14ZX01; China Hepatitis Prevention Foundation - Tianqing Liver Disease Research Foundation, No. TQGB20150104
摘要:
文题释义:
脂肪干细胞:是指从脂肪组织中分离得到的一种间充质干细胞,不但具有跨胚层多向分化潜能,在不同培养条件下可以分化成肌肉、软骨、脂肪组织、神经组织或肝脏组织,而且具备取材方便、来源广阔、增殖能力强、免疫原性低等优点,近年来成为干细胞治疗的热点。
外泌体:是一种细胞主动分泌的大小均一、直径为50-150 nm的脂质双分子层结构囊泡,可由树突细胞、淋巴细胞、成纤维细胞、间充质干细胞和肿瘤细胞等多种不同细胞类型释放。
背景:肝纤维化具有较高的发病率和死亡率,肝星状细胞的活化和增殖是肝纤维化进程中的关键环节。目前还没有针对单一环节或靶点的有效抗纤维化药物。
目的:分析人脂肪干细胞来源外泌体对四氯化碳诱导的大鼠肝纤维化的影响。
方法:①通过酶溶解法获取健康人群来源脂肪中干细胞,体外培养获取一定数量细胞后通过多重超滤法获取外泌体。体外培养的肝星状细胞经转化生长因子β1活化后利用不同浓度外泌体进行处理,通过定量PCR检测细胞内α-平滑肌动蛋白的表达明确其活化程度,以及分别使用CCK-8及流式细胞术检测各组外泌体处理后活化肝星状细胞的生长率及凋亡率。②通过腹腔注射四氯化碳构建肝纤维化大鼠动物模型,尾静脉注射外泌体进行治疗。检测各组动物的肝功能及血清Ⅲ型前胶原、Ⅳ型胶原,肝组织Ishak评分及肝纤维化半定量,以及通过免疫荧光法检测肝组织内基质金属蛋白酶组织抑制剂1、基质金属蛋白酶9及α-平滑肌动蛋白的表达。实验方案于2017年1月经同济大学动物实验伦理委员会以及医学伦理学委员会批准。
结果与结论:人脂肪干细胞来源外泌体可抑制活化的肝星状细胞增殖,其可能的机制为抑制活化巨噬细胞的增殖,减少胶原纤维、α-平滑肌动蛋白及基质金属蛋白酶组织抑制剂1的表达,并促进基质金属蛋白酶9的表达。提示外泌体可治疗四氯化碳诱导肝纤维化。
orcid: 0000-0002-7141-8135 (Li Hongchao)
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中图分类号:
引用本文
李洪超, 王 皙, 李 莉, 李震宇, 臧祖盛, 周 桁, 王晓今, 陈成伟, 程明亮, 吴 君, 金银鹏, 傅青春. 人脂肪干细胞来源外泌体对四氯化碳诱导肝纤维化模型大鼠的治疗作用[J]. 中国组织工程研究, 2020, 24(13): 1996-2004.
Li Hongchao, Wang Xi, Li Li, Li Zhenyu, Zang Zusheng, Zhou Heng, Wang Xiaojin, Chen Chengwei, Cheng Mingliang, Wu Jun, Jin Yinpeng, Fu Qingchun. Therapeutic effect of human adipose stem cells derived exosomes on carbon tetrachloride induced liver fibrosis in rats [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(13): 1996-2004.
Separation
and identification of hASCs and exosomes
Under the inverted
microscope, hASCs were fibrous, uniform in size and grew in an eddy-shaped
manner. Flow cytometry showed that CD molecules on the surface of hASCs were
positive for CD90, CD44, CD105 and CD73, but negative for CD34, CD19 and CD45.
After cultured in lipid-induced medium, hASCs were stained with oil red O. Red
lipid droplets were observed under optical fiber microscopy. After the
differentiation of hASCs was induced by osteogenic induction medium, a large
number of red nodules could be seen in intracellular calcium nodules stained with
Alizarin red.
Under
scanning electron microscopy, exosomes were observed as uniform round cup
shapes with vesicular structures between 30 and 150 nm in size. The diameter of
exosomes was 30-150
nm by Nanosight granulometer. The results of antibody microarray showed that
FLOT1 (flotillin 1), ICAM1 (intercellular adhesion molecule 1), ALIX, CD81,
CD63, EpCAM (epithelial cell adhesion molecule), ANXA5 (annexin A5), TSG101
(tumor susceptibility gene 101) were all positive, while GM130 (cis-Golgi
matrix protein) was negative. Histogram statistical analysis showed that the
gray values of FLOT1, ICAM, ALIX, CD81, CD63, EpCAM, ANXA5 and TSG101 were all
higher than those of the blank group (data not shown).
Effect
of exosomes on proliferation and apoptosis of activated HSCs
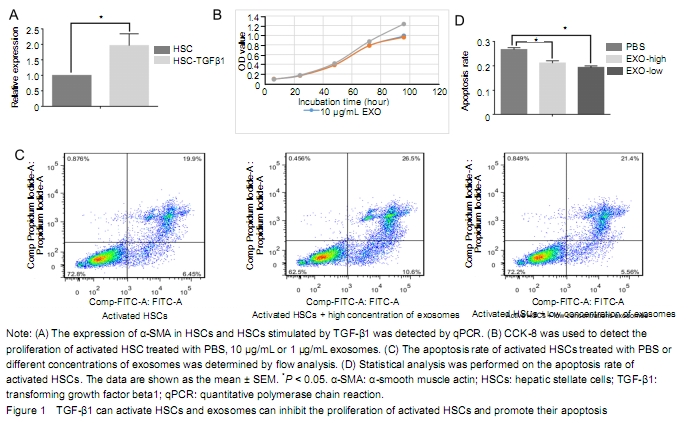
The expression level
of α-SMA in HSCs stimulated by TGF-β1 was significantly higher than that of
HSCs. It indicates that TGF-β1 could activate HSCs (Figure 1A). Compared with the PBS group, the proliferation of
activated HSCs was inhibited by exosome, especially at 96 hours. However, there
was no significant difference between the 10 μg/L and 1 μg/L exosome treatment
groups. It indicates that exosome could inhibit the proliferation of activated
HSC (Figure 1B). As shown in Figure 3C, the late apoptosis
percentage of activated HSCs in the high or low concentration exosomes groups
and control group was 26.5%, 21.4%, and 19.9% respectively, while the early
apoptosis percentage was 10.6%, 5.56%, and 6.45% respectively. The early and
late apoptosis rate of high concentration exosome treatment group was higher
than that of the PBS group. The late apoptosis rate in the low concentration
exosome treatment group was higher than that in the PBS group, while the early
apoptosis rate in the low concentration exosome treatment group was lower than
that of the PBS group (Figure 1C).
Compared with PBS and low-concentration exosome treatment groups, the treatment
of activated HSCs with high concentration exosomes can significantly promote
later apoptosis. It indicates that the apoptosis of activated HSC was
significantly promoted when the exosome concentration reached a certain level (Figure 1D).
Quantitative
analysis of experimental animals
All rats were involved
in the result analysis.
Effect
of exosomes on liver fibrosis
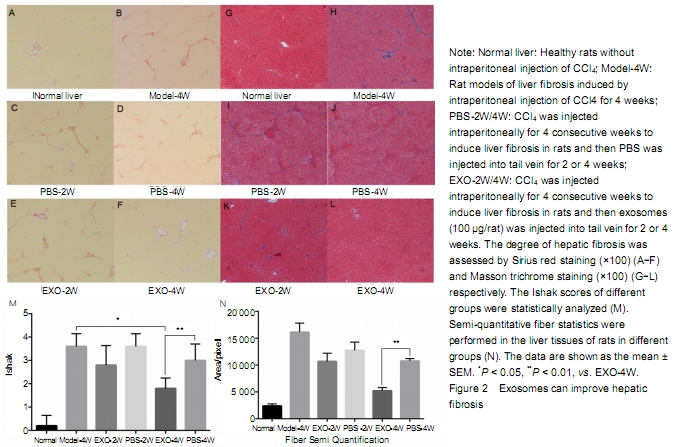
CCl4 was
injected intraperitoneally for 4 weeks to induce liver fibrosis. Sirius red and
Masson trichrome staining of liver tissue showed moderate to severe liver
fibrosis. Continuous tail venous injection of exosomes alleviated liver fibrosis,
with the best effect at 4 weeks compared with the other groups (Figure 2A-L). After
liver staining, Ishak was scored and compared statistically (Figure 2M). After intraperitoneal
injection of CCl4 in rats, Ishak score reached 3-4,
indicating moderate and severe fibrosis has formed. Compared with the rat
models of liver fibrosis without PBS or exosome therapy, the Ishak scores of
PBS treatment at 2 or 4 weeks were insignificantly different, indicating that
the establishment of liver fibrosis model in rats is relatively stable. After 4
weeks of treatment with exosomes, liver fibrosis was significantly reduced.
Semi-quantitative fiber statistics showed that exosomes significantly reduced
the degree of liver fibrosis after 4 weeks of treatment (Figure 2N). No animals died during the experiment.
Effects
of exosomes on serum liver function, type PIII and IV-C collagen in rats with
liver fibrosis
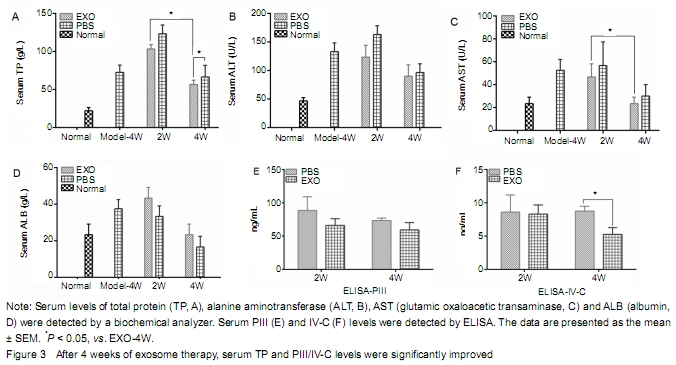
Compared with the
exosome treatment group, serum ALT and AST levels were slightly higher, while
serum TP and ALB levels were lower in the PBS group, indicating that exosome
can improve the liver function. Serum TP level in the EXO-4W group was
significantly higher than that in the PBS-4W group (P < 0.05). However, there was no significant difference in serum
albumin level between exosomes and PBS groups (Figure 3A-D). It suggests that
the effect of exosomes on the recovery of hepatic enzymes is not obvious in
rats with liver fibrosis. Serum PIII and IV-C collagen levels in the exosome
treatment group were lower than those in the PBS group, and there was
statistical significance between EXO-4W and PBS-4W (P < 0.05) (Figure 3E-F). The
results showed that exosomes decreased the expression of serum PIII/IV-C and
alleviated liver fibrosis.
Effect
of exosomes on the ECM protein expression in rats with liver fibrosis
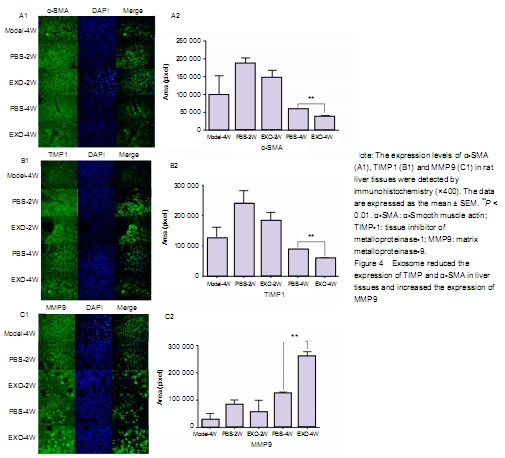
Immunohistochemical
staining showed that the expression of α-SMA and TIMP1 was high in liver tissue
of rats with liver fibrosis, while the expression of MMP9 was low. With the
prolongation of exosome therapy time, the expression of α-SMA and TIMP1 in
liver tissue decreased gradually, and the expression of MMP9 in ECM increased
gradually. There was significant difference in protein expression in ECM
between PBS-4W and EXO-4W. It indicates that exosomes could inhibit activated
HSCs to produce α-SMA and TIMP1, and promote HSCs to produce MMP9.
| [1] LI T, YAN Y, WANG B, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22(6):845-854. [2] MOTAVAF M, PAKRAVAN K, BABASHAH S, et al. Therapeutic application of mesenchymal stem cell-derived exosomes: a promising cell-free therapeutic strategy in regenerative medicine. Cell Mol Biol (Noisy-le-grand). 2016;62(7):74-79. [3] SATO M, SUZUKI S, SENOO H. Hepatic stellate cells: unique characteristics in cell biology and phenotype. Cell Struct Funct. 2003;28(2):105-112. [4] CHEN G, JIN Y, SHI X, et al. Adipose-derived stem cell-based treatment for acute liver failure. Stem Cell Res Ther. 2015;6:40. [5] GRIFFIN MD, RYAN AE, ALAGESAN S, et al. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: what have we learned so far? Immunol Cell Biol. 2013;91(1):40-51. [6] DOMINICI M, LE BLANC K, MUELLER I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8(4):315-317. [7] PITTENGER MF, MACKAY AM, BECK SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284(5411):143-147. [8] TIMMERS L, LIM SK, ARSLAN F, et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007;1(2):129-137. [9] VICKERS KC, REMALEY AT. Lipid-based carriers of microRNAs and intercellular communication. Curr Opin Lipidol. 2012;23(2):91-97. [10] KOUREMBANAS S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 2015;77: 13-27. [11] TÖGEL F, VALERIUS MT, FREEDMAN BS, et al. Repair after nephron ablation reveals limitations of neonatal neonephrogenesis. JCI Insight. 2017;2(2):e88848. [12] KINNAIRD T, STABILE E, BURNETT MS, et al. Bone-marrow-derived cells for enhancing collateral development: mechanisms, animal data, and initial clinical experiences. Circ Res. 2004;95(4):354-363. [13] NAKAGAMI H, MAEDA K, MORISHITA R, et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005;25(12):2542-2547. [14] SHI R, JIN Y, CAO C, et al. Localization of human adipose-derived stem cells and their effect in repair of diabetic foot ulcers in rats. Stem Cell Res Ther. 2016;7(1):155. [15] ASLAM M, BAVEJA R, LIANG OD, et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. 2009;180(11):1122-1130. [16] CHENG K, RAI P, PLAGOV A, et al. Transplantation of bone marrow-derived MSCs improves cisplatinum-induced renal injury through paracrine mechanisms. Exp Mol Pathol. 2013;94(3):466-473. [17] KIM YS, AHN JS, KIM S, et al. The potential theragnostic (diagnostic+therapeutic) application of exosomes in diverse biomedical fields. Korean J Physiol Pharmacol. 2018;22(2):113-125. [18] WANG X, ZHANG H, BAI M, et al. Exosomes serve as nanoparticles to deliver anti-miR-214 to reverse chemoresistance to cisplatin in gastric cancer. Mol Ther. 2018;26(3):774-783. [19] JIN Y, WANG J, LI H, et al. Extracellular vesicles secreted by human adipose-derived stem cells (hASCs) improve survival rate of rats with acute liver failure by releasing lncRNA H19. EBioMedicine. 2018;34:231-242. [20] NI J, LI H, ZHOU Y, et al. Therapeutic potential of human adipose-derived stem cell exosomes in stress urinary incontinence - an in vitro and in vivo study. Cell Physiol Biochem. 2018;48(4):1710-1722. [21] LI R, SONG J, WU W, et al. Puerarin exerts the protective effect against chemical induced dysmetabolism in rats. Gene. 2016;595(2):168-174. [22] WANG JH, CHOI MK, SHIN JW, et al. Antifibrotic effects of Artemisia capillaris and Artemisia iwayomogi in a carbon tetrachloride-induced chronic hepatic fibrosis animal model. J Ethnopharmacol. 2012; 140(1):179-185. [23] VERLOH N, PROBST U, UTPATEL K, et al. Influence of hepatic fibrosis and inflammation: Correlation between histopathological changes and Gd-EOB-DTPA-enhanced MR imaging. PLoS One. 2019;14(5):e0215752. [24] NAGAKI M, SUGIYAMA A, NAIKI T, et al. Control of cyclins, cyclin-dependent kinase inhibitors, p21 and p27, and cell cycle progression in rat hepatocytes by extracellular matrix. J Hepatol. 2000;32(3):488-496. [25] ASEF A, MORTAZ E, JAMAATI H, et al. Immunologic role of extracellular vesicles and exosomes in the pathogenesis of cystic fibrosis. Tanaffos. 2018;17(2):66-72. [26] YONG-HUA Z, XIAN-SHI T, LI-JUAN S, et al. Advances in the regulatory roles of exosomes on pathological process of schistosomiasis hepatic fibrosis. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2018;30(5):596-600. [27] LAI P, CHEN X, GUO L, et al. A potent immunomodulatory role of exosomes derived from mesenchymal stromal cells in preventing cGVHD. J Hematol Oncol. 2018;11(1):135. [28] JIANG W, TAN Y, CAI M, et al. Human umbilical cord MSC-derived exosomes suppress the development of CCl4-induced liver injury through antioxidant effect. Stem Cells Int. 2018;2018:6079642. [29] NIU WH, ZHANG JJ, ZHU ZY. Research advances in the role of mesenchymal stem cells and their exosomes in treatment of liver diseases. Zhonghua Gan Zang Bing Za Zhi. 2017;25(10):793-796. [30] LOU G, CHEN Z, ZHENG M, et al. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med. 2017;49(6):e346. [31] CHEN L, CHARRIER A, ZHOU Y, et al. Epigenetic regulation of connective tissue growth factor by MicroRNA-214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology. 2014;59(3):1118-1129. [32] SEO W, EUN HS, KIM SY, et al. Exosome-mediated activation of toll-like receptor 3 in stellate cells stimulates interleukin-17 production by γδ T cells in liver fibrosis. Hepatology. 2016;64(2):616-631. [33] NING L, ZHU B, GAO T. Gold nanoparticles: promising agent to improve the diagnosis and therapy of cancer. Curr Drug Metab. 2017;18(11):1055-1067. [34] GNECCHI M, DANIELI P, MALPASSO G, et al. Paracrine mechanisms of mesenchymal stem cells in tissue repair. Methods Mol Biol. 2016;1416:123-146. [35] GNECCHI M, HE H, LIANG OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11(4):367-368. [36] PHINNEY DG, DI GIUSEPPE M, NJAH J, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. [37] LI HC, JIN YP, WANG X, et al. Safety of freeze-dried powder of human adipose-derived stem cells and its exosomes. Zhongguo Zuzhi Gongcheng yanjiu. 2018;22(29):4593-4600. [38] WEN D, PENG Y, LIU D, et al. Mesenchymal stem cell and derived exosome as small RNA carrier and Immunomodulator to improve islet transplantation. J Control Release. 2016;238:166-175. [39] ARSLAN F, LAI RC, SMEETS MB, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10(3):301-312. [40] FIORE EJ, MAZZOLINI G, AQUINO JB. Mesenchymal stem/stromal cells in liver fibrosis: recent findings, old/new caveats and future perspectives. Stem Cell Rev. 2015;11(4):586-597. [41] HUANG B, CHENG X, WANG H, et al. Mesenchymal stem cells and their secreted molecules predominantly ameliorate fulminant hepatic failure and chronic liver fibrosis in mice respectively. J Transl Med. 2016;14:45. [42] LI J, GHAZWANI M, ZHANG Y, et al. miR-122 regulates collagen production via targeting hepatic stellate cells and suppressing P4HA1 expression. J Hepatol. 2013;58(3):522-528. [43] CHUN-YAN L, ZI-YI Z, TIAN-LIN Y, et al. Liquid biopsy biomarkers of renal interstitial fibrosis based on urinary exosome. Exp Mol Pathol. 2018;105(2):223-228. [44] QU Y, ZHANG Q, CAI X, et al. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med. 2017; 21(10):2491-2502. [45] OISHI Y, MANABE I. Adipose stem cell system. Clin Calcium. 2017; 27(6):795-801. [46] WANG JY. The study on diagnostic value of co-detection of serum IV-C HA and LN in liver fibrosis patients. Yixue Lilun yu Shjian. 2001;14(2):106-108. |
| [1] | 蒲 锐, 陈子扬, 袁凌燕. 不同细胞来源外泌体保护心脏的特点与效应[J]. 中国组织工程研究, 2021, 25(在线): 1-. |
| [2] | 陈继铭, 吴晓静, 刘田丰, 陈海聪, 黄成硕. 水飞蓟素对四氯化碳致小鼠肝损伤和骨代谢的影响[J]. 中国组织工程研究, 2021, 25(8): 1224-1228. |
| [3] | 赵 敏, 冯柳祥, 陈 垚, 顾 霞, 王平义, 李一梅, 李文华. 低氧环境下外泌体可作为疾病的标志物[J]. 中国组织工程研究, 2021, 25(7): 1104-1108. |
| [4] | 马泽涛, 曾 晖, 王德利, 翁 鉴, 冯 松. 微小RNA-138-5p与软骨细胞增殖和自噬的关系[J]. 中国组织工程研究, 2021, 25(5): 674-678. |
| [5] | 聂慧娟, 黄织春. 转化生长因子β1诱导心肌成纤维细胞转分化过程中Hedgehog信号通路的作用机制[J]. 中国组织工程研究, 2021, 25(5): 754-760. |
| [6] | 陈子扬, 蒲 锐, 邓 爽, 袁凌燕. 外泌体对运动介导胰岛素抵抗类疾病的调控作用[J]. 中国组织工程研究, 2021, 25(25): 4089-4094. |
| [7] | 郝晓娜, 张英杰, 李玉云, 许 涛. 过表达脯氨酰寡肽酶的骨髓间充质干细胞修复肝纤维化模型大鼠[J]. 中国组织工程研究, 2021, 25(25): 3988-3993. |
| [8] | 高 坤, 陈大宇, 张 勇, 刘伟东, 孙淑芬, 赖文强, 马笃军, 吴益宏, 林展鹏, 蒋鹰鹭, 余伟吉. 牛膝醇提物调控滑膜成纤维细胞外泌体抑制软骨细胞外基质降解[J]. 中国组织工程研究, 2021, 25(23): 3636-3640. |
| [9] | 杨 莉, 李雪莉, 宋静卉, 禹卉千, 王伟霞. 隐丹参酮抑制模型兔耳增生性瘢痕的作用及机制[J]. 中国组织工程研究, 2021, 25(20): 3150-3155. |
| [10] | 赵双丹, 郑嘉华, 亓文博, 黄向华. 间充质干细胞外泌体应用于生殖系统疾病治疗的作用与机制[J]. 中国组织工程研究, 2021, 25(19): 3097-3102. |
| [11] | 严秀蕊, 陶 金, 梁雪云. 人胎盘间充质干细胞来源外泌体保护氧化应激损伤肺上皮细胞的机制[J]. 中国组织工程研究, 2021, 25(19): 2994-2999. |
| [12] | 王 康, 智晓东, 王 伟. 干细胞来源外泌体修复周围神经损伤的效应[J]. 中国组织工程研究, 2021, 25(19): 3083-3089. |
| [13] | 刘 冯, 张 瑜, 王燕丽, 骆 威, 韩超珊, 李杨欣. 温敏型壳聚糖水凝胶包封外泌体在缺血性疾病中的应用[J]. 中国组织工程研究, 2021, 25(16): 2479-2487. |
| [14] | 刘云逸, 王 博, 王 琳. 运动后针刺腓肠肌干预肥胖大鼠肌腱退行性病变[J]. 中国组织工程研究, 2021, 25(14): 2211-2218. |
| [15] | 蔡 原, 邓呈亮. 脂肪干细胞无细胞提取液的治疗用途:皮肤老化、创面愈合、瘢痕恢复乃至神经再生[J]. 中国组织工程研究, 2021, 25(13): 2097-2102. |
Liver
fibrosis is a chronic liver injury characterized by excessive extracellular
matrix (ECM) in liver tissue and caused by a variety of factors including viral
hepatitis, drugs and autoimmune diseases[1]. All kinds of pathogenic
factors can cause hepatocyte damage, necrosis and inflammatory reaction,
activate Kupffer cells in the liver, and secrete tumor necrosis factor alpha
(TNF-α), transforming growth factor beta (TGF-β), interleukin (IL) 1β and IL-6.
All cytokines can activate hepatic stellate cells (HSCs) toward myofibroblasts.
Myofibroblasts accelerate the synthesis and deposition of ECM, eventually
leading to liver fibrosis. ECM includes α-smooth muscle actin (α-SMA), tissue
inhibitor of metalloproteinase-1 (TIMP-1), and matrix metalloproteinase-9
(MMP9)[2]. The activation of HSCs and inflammation are important
factors contributing to the occurrence and development of liver fibrosis[3].
Therefore, promoting the apoptosis of activated HSCs and inhibiting the
production of inflammatory factors are of great significance for the treatment
of liver fibrosis.
Human adipose derived mesenchymal stem cells (hASCs), which have strong self-renewal capacity, multidirectional differentiation potential across the dermal layer and have advantages of relatively easy collection of sources and higher proliferation rate, are expected to treat liver fibrosis[4-7]. Multiple studies have shown that hASCs can alleviate liver fibrosis by promoting the apoptosis
of activated HSCs and reducing the production of fibers[2, 8]. hASCs can secrete exosomes, which have abundant proteins, lipids and RNA, into the extracellular space for intercellular communication.
Exosomes, which can be actively secreted by various cells, are lipid bilayer structure vesicles with the diameter of 30-150 nm[9]. Double membrane structure of exosomes can protect the substances from being degraded by various enzymes outside the cells. Exosomes play a therapeutic role by binding to the receptor expressed on the surface of the target cells[10-14]. At present, several research groups have established that the exosomes isolated from different stem cell conditioned medium can alleviate liver or kidney fibrosis in animals. Some scholars have found that HSCs in resting state contain relatively abundant miR-214, which can inhibit the expression of CCN2 protein by binding 3'UTR of CCN2 gene after being transferred to nearby HSCs or hepatocytes through exosomes. In addition, some studies have found that in the early stage of liver fibrosis, exosomes can mediate the activation of Toll-like receptor 3 (TLR3) of HSCs, promote the secretion of IL-17A, CCL20, IL-1beta, IL-23 and other factors by HSCs, and then promote liver fibrosis by increasing the production of IL-17A in γδT cells. It can be seen that exosomes can reduce the production of collagen I and III and alleviate the fibrosis by promoting the apoptosis of activated HSCs and decreasing the expression of inflammatory cytokines[15-18]. Also, exosomes can inhibit inflammation and immune response, and promote hepatocyte regeneration and angiogenesis in rats with liver failure[4, 14, 19-20]. However, there are few studies regarding the mechanism by which exosome exhibits effects on liver fibrosis. This study explored the effect of hASCs derived exosomes on rats with liver fibrosis induced by carbon tetrachloride (CCl4). Indicators of liver fibrosis were tested, including MMP9, α-SMA, TIMP-1, type III precollagen (PCIII), type IV collage (IV-C) and the apoptosis of activated HSCs.
Design
A randomized controlled animal experiment.
Time and setting
The experiment was conducted in the Key Laboratory of Stem Cell Physiology and Disease Treatment Research Group, Department of Regenerative Medicine, Tongji University, Shanghai from January 2017 to June 2018.
Materials
Experimental animals: 4- to 6-week-old male specific-pathogen-free Sprague-Dawley rats (n = 36) were provided by Shanghai Bikai Experimental Animals Co., Ltd., and included in this study. All animals were allowed free access to food and water in the Laboratory Animal Center of Tongji University, China. Animal experiments had been approved by the Animal Ethics Committee of the 905 Hospital of PLA Navy, China.
Laboratory reagents: 0.1% collagenase I (Gibco, USA), F12/DMEM (Gibco, USA), fetal bovine serum (Gibco, USA), penicillin-streptomycin (Gibco, USA), anti-human CD44/CD90/CD73/CD19/CD34/CD45-FITC, CD105-PE antibodies (Bioscience, USA), adipogenic or osteogenic differentiation medium (Cyagen Biosciences, China), oil red (Sigma, USA), alizarin red (Sigma, USA), exosomes’ surface molecule antibody chips (System Biscience, USA), TGF-1 factor (Pepro Tech, USA), TRIzol (Aidlab, beijing, China), RT reagent kit (Tiagen, Beijing, China), CCK8 (Dongren, Shanghai, China), apoptosis kit (BD, USA), 4% paraformaldehyde (Dingguo Changshen, Beijing, China), Sirius red and Masson trichrome stain (Solaibao, Beijing, China), ELISA kit (Solaibao), gradient alcohol (Tansoole, Shanghai, China), TIMP1/MMP9/SMA primary antibody (CST, USA), secondary antibody (SCT, USA), DAPI (Solarbio).
Experimental instrument: centrifuge (TD25-WS, Lu Xiangyi, China), incubator (Thermo, USA), flow cytometer (Caliber, BD), ultrafiltration enrichment centrifuge tube (Merk, Ireland), Nanosight granulometer (NS300, Britain), multifunctional enzyme marker (SoftMax Pro5, Molecular Devices, USA), automatic analyzer (Hitachi, Tokyo, Japan), microscopic examination (IX71, Olympus, Tokyo, Japan), Xylene (Merk, Ireland), and fluorescence microscope (IX71).
Methods
Separation and identification of hASCs and exosomes
Separation of hASCs: Harvesting of healthy human adipose tissue was approved by the Medical Ethics Committee of Tongji University, China in January, 2017. All patients provided written informed consent prior to the inception of the study. Healthy human adipose tissue was harvested from the subcutaneous portion of the neck provided by the 85th Hospital of Chinese PLA. After three PBS washes, human adipose tissue was chopped into small blocks with tissue scissors, digested by type I collagenase for 45-60 minutes, and centrifuged at 1 200 r/m for
5 minutes. The resultant cells were incubated in F12/DMEM supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin in an incubator at 37 oC with 5% CO2. Cell density was 80% for these experiments.
Identification of hASCs: hASCs were prepared as single cell suspension in seven tubes. Anti-human CD44/CD90/ CD73/CD19/CD34/CD45-FITC, CD105-PE antibodies were added separately. Then the mixture was incubated at 37 oC for 30 minutes and flow cytometry was performed. hASCs were cultured in lipogenic or osteogenic differentiation medium for 3 weeks and stained with oil red and alizarin red, respectively.

Separation and identification of exosomes: When hASCs density reached 80%, the medium containing serum was removed and replaced with basal medium for 24 hours. The supernatant was collected and centrifuged at 4 000 × g at 4 oC for 40 minutes in ultrafiltration enrichment centrifuge tube. Then concentrate was collected and stored at -80 oC. Exosome size was
measured using
scanning electron microscope and Nanosight granulometer. Exosome surface
molecules of FLOT1 (Flotillin 1), ICAM (intercellular adhesion), ALIX, CD81,
CD63, EpCAM (epithelial cell adhesion molecule), ANXA5 (annexin A5) and TSG101 (tumor
susceptibility gene 101) were detected by antibody chips.
Cell culture and
treatments
Activation of HSCs: At 70-80%
confluence, HSCs were inoculated in 6-well plates and treated with TGF-1 at a
final concentration of 10 ng/mL or with PBS for 24 hours. Then, medium was
removed and total RNA of activated HSC was extracted with TRIzol and then
reverse transcribed to complementary DNA using a Primescript TM RT reagent kit.
qPCR was then performed using SYBR Green qPCR Super Mix-UDG on a Step One Plus
Real-Time PCR System. The relative abundance of the target genes was determined
by the comparative cycle threshold Ct method (2-ΔΔCt) and
normalized to GAPDH.
Proliferation of HSCs: Activated
HSCs were inoculated in 96-well plates at 2 000 cells and treated with high
concentration (10 μg/mL) or low concentration (1 μg/mL) exosomes. CCK8 was used
to detect activated HSCs’ proliferation according to the manufacturer’s
instructions and multifunctional enzyme marker was used to measure optical
density value at 450 nm.
Apoptosis of HSCs: Activated
HSCs were inoculated in a medium containing 0.01 μL/mL CCl4 for 24 hours and
treated with different concentrations of exosomes (10 and 1 μg/mL). Apoptosis
was detected according to the manufacturer’s instructions (BD, USA).
Establishing rat
models of liver fibrosis
Liver fibrosis was induced by intraperitoneal injection of CCl4 (reconstituted in olive oil at a ratio of 1∶5 and administered at a dose of 2 mL/kg body weight) twice weekly for 4 consecutive weeks[21-22]. There are six groups in animal experiments (6 rats/group): A: normal animal; B: modeling for 4 weeks; C: modeling for 4 weeks + exosome therapy for 2 weeks (100 μg/rat); D: modeling for 4 weeks + PBS therapy for 2 weeks (100 μg/rat); E: modeling for 4 weeks + exosome therapy for 4 weeks (100 μg/rat); F: modeling for 4 weeks + PBS therapy for 4 weeks (100 μg/rat).
Exosomes treatment of
liver fibrosis in rats
Exosomes (administered
at a volume of 200 μL, 100 μg/rat) or PBS (administered at a dose of 200 μL)
treatment was performed by tail vein twice weekly in rats 4 weeks after
induction of liver fibrosis for 2 weeks or 4 weeks. Blood samples were
centrifuged at 3 000×g for 10 minutes
at 4 oC, and then serum was collected and stored at -80 oC
until use. A section of hepatic tissue from the porta hepatis was fixed in 4%
paraformaldehyde and the remaining hepatic tissue was preserved at -80 oC.
Serum liver enzyme
detection
Serum total protein
(TP), albumin (ALB), alanine aminotransferase (ALT) and aspartate
aminotransferase (AST) were assayed by an automatic analyzer.
Assessment of liver
fibrosis
Paraformaldehyde-fixed
samples were embedded in paraffin, sectioned (4-μm thickness), and stained with
Sirius red and masson trichrome for microscopic examination. The liver fibrosis
in rats was evaluated by Ishak score standard[23]. Image-pro Plus
6.0 software was used for semi-quantitative analysis of liver tissue images
stained with Sirius red and masson trichrome. ELISA was performed to detect
serum levels of PCIII and IV-C. Hepatic tissue embedded by paraffin was
conventionally dewaxed by xylene and gradient alcohol. Then primary antibody
was added after antigenic repair and serum closure. The sections were incubated
overnight at 4 oC and then incubated with a suitable
fluorescently-conjugated (FITC) secondary antibody. DAPI was used for nuclear
counterstaining. TIMP-1, MMP-9 and α-SMA expression in rat liver tissue was
evaluated under the fluorescence microscope. At the same time, Image-pro Plus
6.0 software was used for semi-quantitative analysis of immunohistochemical
fluorescence images.
Main
outcome measures
Expression of TIMP-1,
MMP-9 and α-SMA in rat liver tissue, ISHAK score and serum levels of PCIII and
IV-C.
Statistical
analysis
All analyses were
performed using Office Excel 2003. The variables were expressed as the mean ±
standard deviation. The comparison between two independent groups was performed
using Student’s t-test. A P-value of 0.05 or less was considered
statistically significant.
Liver fibrosis is the liver’s response to chronic inflammation, necrosis, or other damages. Liver failure or liver cancer caused by liver fibrosis is the leading cause of death. The continuous activation of HSCs is a key link in the development of liver fibrosis. The proliferation, adhesion, and migration of activated HSCs will increase the expression of type I and III collagen and reduce
degradation in EMS. Increasing the apoptosis and collagen degradation of
activated HSCs can significantly improve or reverse liver fibrosis[24].
Current treatments for liver fibrosis include etiological therapy, antifibrotic drugs, orthotopic liver transplantation, cell-based therapy and traditional Chinese medicine. These treatments mainly focus on etiological treatment, inhibition of inflammation, regulation of HSCs, regulation of other liver immune cells, regulation of cell receptor-ligand interactions related to liver fibrosis, correction of the imbalance between ECM production and degradation and probiotics[25-27].
However, due to the complexity of the etiology and pathogenesis of liver
fibrosis, the limitations of anti-etiology and fibrosis drugs, the lack of
liver source and the potential of liver injury of Chinese herbal medicine, cell
therapy has been widely studied. At present, cell therapy is mainly based on
the ability of MSCs to differentiate into multiple tissues. However, the immune
rejection of MSCs derived from various tissues remains a major problem when
transplanted into damaged organisms, which limits the use of cell therapy due
to safety and other problems. MSCs play a therapeutic role by producing some
bioactive factors, and the exosome is one of the most important substances[28-30].
In this study, multiple ultrafiltration concentration method was used to
obtain hASCs derived exosomes for treatment (Ultrafiltration method of
extracting exosomes with 100 KD filter membrane at 4 000 × g per min was invented by our research group. It conforms to the
characteristics of exosomes and has applied for a patent. The patent number is
CN201710447410.4). Exosomes contain rich RNA, microRNA and protein components,
which can exchange information with target cells, promote cell proliferation,
migration or inhibit cell apoptosis, and thus promote tissue damage repair. For
example, stem cell-derived exosomes inhibit the release of CX3CL1 macrophage
chemoattractant protein and increase the expression of IL-10 to repair damage[31-32].
In the previous experiments, our research group carried out hemolytic
test, vascular stimulation test, muscle stimulation test, active systemic
allergy test, passive skin allergy test, and other safety tests on hASCs
derived exosomes. The results showed that hASCs derived exosomes obtained by
ultrafiltration concentration method were safe for in vivo injection[9, 33-37].
TGF-β1, which is the most abundant member of TGF-β1 family in cells and
tissues, can activate HSCs. Activated HSCs expressing α-SMA, vimentin and
desmin transformed into myofibroid mother cells[36, 38-43].
Activated HSCs can promote mass synthesis of ECM and accelerate the progression
of liver fibrosis[44-45]. Inhibiting the proliferation or promoting
the apoptosis of activated HSCs can inhibit the progression of liver fibrosis
and even alleviate liver fibrosis. In
vitro, we found that the activated HSCs treated by high concentration of exosomes
could significantly inhibit cell proliferation at 96 hours, and promote early
and late apoptosis of activated HSCs. In summary, exosomes can promote the
apoptosis of activated HSCs and alleviate liver fibrosis.
Serum type III and IV collagen is one of the
commonly used non-invasive detection indicators reflecting liver fibrosis. It
is mainly synthesized and released by HSCs, and it is the main component in the
matrix of liver connective tissue. It is considered as a more sensitive
detection indicator to judge the degree of liver fibrosis[46]. In
this study, serum PIII and IV-C level of rats with hepatic fibrosis was
detected after 2 or 4 weeks of treatment with exosome or PBS. Serum PIII and
IV-C expression of EXO-4W rats was decreased by exosomes in the same cycle
treatment group, and serum PIII/IV-C expression in EXO-4W rats was
significantly different from that of PBS-4W rats. In addition, serum PIII/IV-C
expression decreased gradually with the increase of exosome treatment time. The
results suggest that exosomes can reduce the production of collagen fibers and
inhibit the progression of liver fibrosis.
Both Sirius red and Masson's trichrome staining of liver tissues indicate
that liver fibrosis in rats treated with exosomes was milder than that in the
PBS group. The Ishak score of liver tissues and semi-quantitative analysis of
stained tissues by Image-pro Plus 6.0 software further confirmed that exosomes
could alleviate liver fibrosis, and there was significant difference between
EXO-4W and PBS-4W rats. Longer exosome treatment indicates stronger effects on
alleviating liver fibrosis.
Immunohistochemical staining showed that exosome inhibits TIMP1/SMA
expression in liver tissue and increases MMP9 expression. The results suggest
that exosomes can alleviate the progression of liver fibrosis by reducing the
production of ECM. In addition, exosomes can alleviate liver fibrosis by
increasing the expression of collagen degradation-related enzymes in ECM. The
degradation of ECM in liver tissue mainly depends on the regulation of
endogenous MMPs and TIMPs. HSCs can secrete a variety of collagenase and matrix
metalloproteinases to degrade various ECM, while activated HSCs promote the
development of liver fibrosis by promoting their own mitosis through autocrine
TIMP-1. Meanwhile, TIMPs bind to specific active collagenase MMP9, which mainly
degrades type IV collagen and denatured collagen, to inhibit the degradation of
ECM.
Combined with the experimental results in vivo and in vitro, we speculate that exosome inhibits the progression of live fibrosis or treats liver fibrosis through inhibiting the proliferation and promoting the apoptosis of activated HSCs, decreasing the number of activated HSCs in liver tissue, and lowering TIMP1 expression in ECM. The expression of MMP9, which can degrade collagen fibers produced by inactivated HSCs, is gradually enhanced, and the production and degradation of collagen fibers in ECM of liver tissue are gradually restored to equilibrium. However, in this experiment, the degradation of TIMP1, MMP9 and α-SMA mRNA in liver tissues by exosomes was not studied intuitively, which will be further confirmed in subsequent experiments.
文题释义:
脂肪干细胞:是指从脂肪组织中分离得到的一种间充质干细胞,不但具有跨胚层多向分化潜能,在不同培养条件下可以分化成肌肉、软骨、脂肪组织、神经组织或肝脏组织,而且具备取材方便、来源广阔、增殖能力强、免疫原性低等优点,近年来成为干细胞治疗的热点。
外泌体:是一种细胞主动分泌的大小均一、直径为50-150 nm的脂质双分子层结构囊泡,可由树突细胞、淋巴细胞、成纤维细胞、间充质干细胞和肿瘤细胞等多种不同细胞类型释放。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
外泌体是目前为止定义最明确的囊泡。细胞内的内溶酶体微粒内陷形成多囊泡体,在刺激作用下,多囊泡体与细胞膜融合,向胞外分泌的大小均一,直径在50-150 nm的囊泡,即为外泌体。外泌体的形成和释放涉及内吞体分选转运体和蛋白。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||