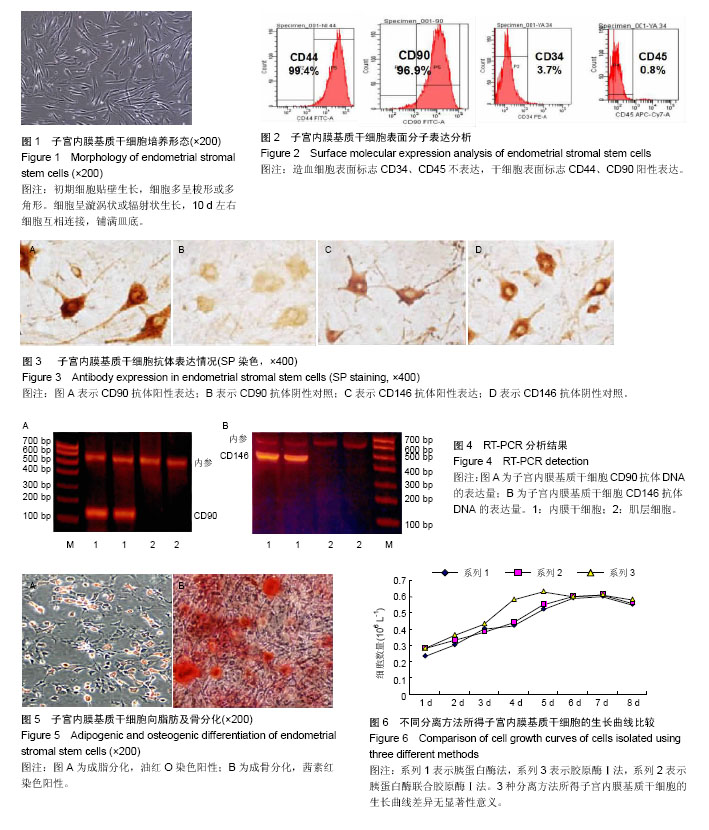
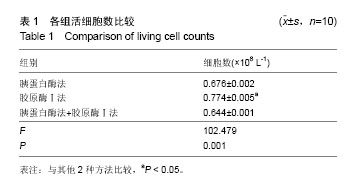
| [1] 高红艳,陈继明,何援利,等.人子宫内膜基质细胞的分离纯化和体外培养方法研究[J].重庆医学,2014,10(34):4634-4636.[2] Deane JA, Gualano RC, Gargett CE, et al. Regenerating endometrium from stem/progenitor cells: Is it abnormal in endometriosis, Asherman's syndrome and infertility? Curr Opin Obstet Gynecol. 2013;25(3):193-200. [3] Ding L,Li X,Sun H, et al.Transplantation of bone marrow mesenchymal stem cells on collagen scaffolds for the functional regeneration of injured rat uterus.Biomaterials. 2014;35(18):4888-4900.[4] Indumathi S,Harikrishnan R,Rajkumar JS,et al.Prospective biomarkers of stem cells of human endometrium and fallopian tube compared with bone marrow. Cell Tissue Res. 2013; 352(3):537-549.[5] Jing Z,Qiong Z,Yonggang W, et al. Rat bone marrow mesenchymal stem cells improve regeneration of thin endometrium in rat. Fertil Steril. 2014;101(2):587-594.[6] Cervelló I, Mas A, Gil-Sanchis C, et al. Somatic stem cells in the human endometrium.Semin Reprod Med. 2013;31(1): 69-76.[7] Yang XY, Wang W, Li X, et al.In vitro hepatic differentiation of human endometrial stromal stem cells. In Vitro Cell Dev Biol. 2014;50(2):162-170.[8] 张继雯.人类子宫内膜肿瘤干细胞的研究进展[J].国际妇产科学杂志,2013,40(4):327-330.[9] Gil-Sanchis C, Cervelló I, Mas A, et al. Leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5) as a putative human endometrial stem cell marker. Mol Hum Reprod. 2013;19(7/8):407-414.[10] Apostolou G, Apostolou N, Biteli M, et al. Utility of Ki-67, p53, Bcl-2, and Cox-2 biomarkers for low-grade endometrial cancer and disordered proliferative/benign hyperplastic endometrium by imprint cytology. Diagn Cytopathol. 2014;42(2):134-142.[11] Chen YZ,Wang JH, Yan J, et al. Increased expression of the adult stem cell marker Musashi-1 in the ectopic endometrium of adenomyosis does not correlate with serum estradiol and progesterone levels. Eur J Obstet Gynecol Reprod Biol. 2014; 173:88-93.[12] Bures N, Nelson G, Duan Q, et al. Primary squamous cell carcinoma of the endometrium: Clinicopathologic and molecular characteristics. Int J Gynecol Pathol. 2013;32(6): 566-575.[13] Mingels MJ, Geels YP, Pijnenborg JM, et al. Histopathologic assessment of the entire endometrium in asymptomatic women. Human Pathol.2013;44(10):2293-2301.[14] 朱明,肖南,黄宏,等.间充质干细胞治疗皮肤创伤的研究进展[J]. 中国细胞生物学学报, 2015,3(6):911-918.[15] 郭珊珊,沈志森,陆达锴,等.干细胞向骨骼肌细胞的诱导分化及其在治疗骨骼肌病变中的应用[J].中国细胞生物学学报, 2015, 4(1):124-131.[16] Ayd?n A, Duruksu G, Erman G, et al. Neurogenic differentiation capacity of subacromial bursal tissue - Derived stem cells. J Orthop Res. 2014;32(1):151-158.[17] Baulcha JE.干细胞释放的微囊泡有望更安全地治疗大脑放射损伤[J].生物医学工程与临床, 2016,11(3):22-23.[18] 于文竹.人胚胎干细胞向子宫内膜上皮样细胞诱导分化的相关研究[D].郑州大学, 2014. [19] Bae D, Moon SH, Park BG, et al. Nanotopographical control for maintaining undifferentiated human embryonic stem cell colonies in feeder free conditions.Biomaterials. 2014;35(3):916-928.[20] Wu JC, Tzanakakis ES. Deconstructing stem cell population heterogeneity: Single-cell analysis and modeling approaches. Biotechnol Adv. 2013;31(7):1047-1062.[21] Arefeh G, Hossein H, Abbas P, et al. Galactosylated collagen matrix enhanced in vitro maturation of human embryonic stem cell-derived hepatocyte-like cells. Biotechnol Lett.2014; 36(5): 1095-1106.[22] Sanjuan-Pla A, Macaulay IC, Jensen CT, et al. Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy.Nature. 2013;502(7470):232-236.[23] Kajihara T, Brosens J, Ishihara O, et al.The role of FOXO1 in the decidual transformation of the endometrium and early pregnancy. Med Mol Morphol. 2013;46(2):61-68.[24] Parra-Herran CE, Yuan L, Nucci MR, et al. Targeted development of specific biomarkers of endometrial stromal cell differentiation using bioinformatics: The IFITM1 model. Mod Pathol. 2014;27(4):569-579.[25] Li J, Hansen KC, Zhang Y, et al. Rejuvenation of chondrogenic potential in a young stem cell microenvironment. Biomaterials. 2014;35(2):642-653.[26] Emmert MY, Wolint P, Wickboldt N, et al. Human stem cell-based three-dimensional microtissues for advanced cardiac cell therapies.Biomaterials. 2013;34(27):6339-6354.[27] Samborski A, Graf A, Krebs S, et al.Transcriptome changes in the porcine endometrium during the preattachment phase. Biol Reprod. 2013;89(6):134-134.[28] Parra-Herran CE, Monte NM, Mutter GL. Endometrial intraepithelial neoplasia with secretory differentiation: Diagnostic features and underlying mechanisms. Mod Pathol. 2013;26(6):868-873.[29] Thiruchelvam U, Dransfield I, Saunders PT, et al. The importance of the macrophage within the human endometrium. J Leukoc Biol. 2013;93(2):217-225.[30] 周华,宋丽娜,张敏.人子宫内膜干细胞体外分离、培养及鉴定[J]. 中华中医药学刊, 2014,17(8):1907-1908.[31] Sakr S, Naqvi H, Komm B, et al. Endometriosis impairs bone marrow-derived stem cell recruitment to the uterus whereas bazedoxifene treatment leads to endometriosis regression and improved uterine stem cell engraftment. Endocrinology. 2014;155(4):1489-1497.[32] 陈岩,李栋,张哲,等. 人脐带间充质干细胞对大鼠子宫内膜异位症病灶神经纤维的影响[J].山东大学学报(医学版),2013,51(3): 48-51.[33] Osuga Y, Koga K, Tsutsumi O, et al. Stem cell factor (SCF) concentrations in peritoneal fluid of women with or without endometriosis. Am J Reprod Immunol. 2000;44(4):231-235. [34] León M, Vaccaro H, Alcázar JL, et al. Extended transvaginal sonography in deep infiltrating endometriosis: Use of bowel preparation and an acoustic window with intravaginal gel: Preliminary results. J Ultrasound Med. 2014;33(2):315-321.[35] 蒋智.人子宫内膜干细胞通过旁分泌作用保护心肌并促进心肌再生改善大鼠心梗后心功能[D].浙江大学, 2013.[36] Jeschke U, Hutter S, Heublein S, et al. Expression and function of galectins in the endometrium and at the human feto-maternal interface. Placenta. 2013;34(10):863-872.[37] 严琰,东健沣,桑运霞,等.经血源子宫内膜干细胞培养、鉴定及体外分化潜能的研究[J].中国细胞生物学学报, 2014,22(7):892- 899.[38] 张倩媚,马颖.子宫内膜干细胞体外诱导及定性分化的研究[J]. 山西医药杂志, 2013, 42(7):770-771.[39] Adammek M, Greve B, Kässens N, et al. MicroRNA miR-145 inhibits proliferation, invasiveness, and stem cell phenotype of an in vitro endometriosis model by targeting multiple cytoskeletal elements and pluripotency factors. Fertil Steril. 2013;99(5):1346-1355. |
.jpg)
.jpg)