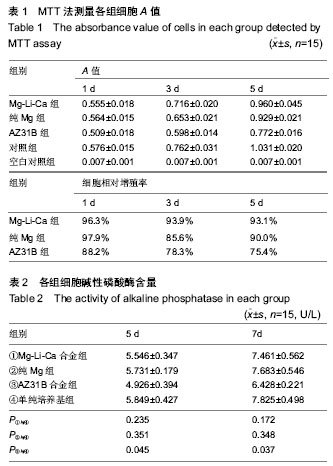
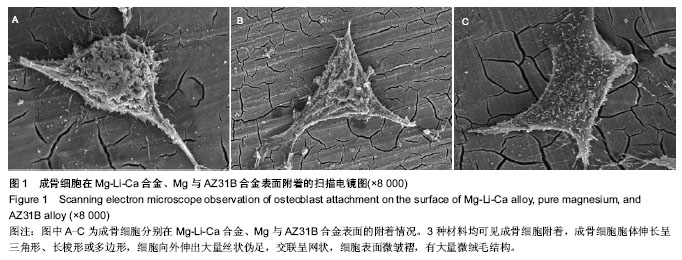
| [1] 张益下颌骨骨折治疗[M].北京:北京医科大学中国协和医科大学联合出版社,1993:76-76.
[2] Wolf FI, Cittadini A.Chemistry and biochemistry of magnesium.Mol Aspects Med. 2003;24(1-3):3-9.
[3] Zreiqat H,Howlett CR,Zannettino A,et al.Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants.J Biomed Mater Res. 2002;62(2):175-184.
[4] Nagels J,Stokdijk M,Rozing PM.Stress shielding and bone resorption in shoulder arthroplastyJ Shoulder Elbow Surg. 2003;12(1):35-39.
[5] 李霄,吴宏流,张绍翔,等.生物医用镁合金研究的文献统计分析[J].生物骨科材料与临床研究,2013,10(4):27-31.
[6] Serre CM,Papillard M,Chavassieux P,et al.Influence of magnesium substitution on a collagen apatite biomaterial on the production of a calcifying mat rix by human osteoblasts.J Biomed Mater Res.1998;42:626.
[7] Kuwahara H,Alabdullat Y,Mazaki N,et al.Precipitation of magnesium apatite on pure magnesium surface during im2 mersing in Hank' s solution.Mater Trans.2001;42:1317.
[8] 高家诚,胡德,宋长江.医用镁合金降解及其对人体的影响[J].功能材料,2012,43(13):1730-1732.
[9] Kirkland NT,Birdilis N,Staiger MP.Assessing the Corrosion of biodegradable magnesium implants:a critical Review of current methodologies and their limitations.Acta Biomaterialia. 2012;8(3):925-936.
[10] Song GL,Zeng RC.Preface for the special issue on light metals as biomaterials. Front Mater Sci.2014;8:199.
[11] Withe F,Ulrich H,Palm C,et al.Biodegradable magnesium scaffolds:PanII:peri-implant bone remodeling.J Biomed Mater Res A.2007;8 1(3):757-765.
[12] Witte F,Kaese V,Haferkamp H,et al.In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials.2005;26:3557-3563.
[13] 曾荣昌,郭小龙,刘成龙,等.医用Mg-Ca和Mg-Li-Ca合金腐蚀研究[J].金属学报,2011, 47(11):1477-1482.
[14] 曹富荣,丁桦,李英龙,等.超轻双相镁锂合金的超塑性、显微组织演变与变形机理[J].中国有色金属学报,2009,19(11): 1908-1916.
[15] Yang X,Yin Q,Zhang Y,et al. Biocompatibility of silicon containing micro-arc oxidation coated magnesium alloy ZK60 with osteoblasts cultured in vitro. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi.2013;27(5):612-618.
[16] Marasco SF,Liovic P,Sutalo ID.Structural integrity of intramedullary rib fixation using a single bioresorbable screw.J Trauma Acute Care Surg.2012;73(3): 668-673.
[17] Wang J,Qin L,Wang K,et al.Cytotoxicity studies of AZ31D alloy and the effects of carbon dioxide on its biodegradation behavior in vitro. Mater Sci Eng C Mater Biol Appl.2013;33(7): 4416-4426.
[18] Guo Y,Ren L,Liu C,et al.Effect of implantation of biodegradable magnesium alloy on BMP-2 expression in bone of ovariectomized osteoporosis rats. Mater Sci Eng C Mater Biol Appl.2013;33(7):4470-4474.
[19] SANKAR J.NSF engineering research center for revolutionizing Metallic biomaterials[EB/OL].http://erc.ncat.edu. 201l;7.
[20] Baril G, Pébère N.The corrosion of pure magnesium in aerated and deaerated sodium sulphate solutions. Corrosion Science.2001;43 (3):471-484.
[21] 崔新战,黄霞,关绍康,等.高分子材料涂覆生物镁合金心血管支架的研究与应用[J].中国组织工程研究,2012,16(51):9635-9639.
[22] 盛明纯.铝对人体健康影响的研究进展综述[J].安徽预防医学杂志,2006,12 (1):46-48.
[23] 孙中蕾,陈瑶,白静.铝中毒研究进展[J].医学综述,2013,15(18): 2741-2743.
[24] 仲志国,卢志文,赵亚忠.镁锂合金的研究进展[J].材料热处理技术,2012,41(4):76-79.
[25] Li Z,Gu X,Lou S,et al.The development of binary Mg-Ca alloysfor use as biodegradable materials within bone. Biomaterials.2008;29(10):1329-1344.
[26] Gu XN,Zheng W,Cheng Y,et al.A study on alkaline heat treated Mg-Ca alloy for the control of the biocorrosion rate. Acta Biomaterialia.2009;5(7):2790-2799.
[27] Liu CL,Wang YJ, Zeng RC,et al.In vitro corrosion degradation behavior of Mg-Ca alloy in the presence of albumin.Corros Sci.2010;52(10):3341-3347.
[28] Zheng YF,Gu XN,Xi YL,et al.In vitro degradation and cytotoxicity of Mg/Ca composites produced by powder metallurgy. Acta Biomaterialia.2010;6(5):1783-1791.
[29] 曾荣昌,郭小龙,刘成龙,等. 医用Mg-Ca和Mg-Li-Ca合金腐蚀研究[J].金属学报,2011,47(11):1477-1482.
[30] Zeng RC,Sun L,Zheng YF,et al.Corrosion and characterisation of dual phase Mg-Li-Ca alloy in Hank’s solution: the influence of microstructural features. Corros Sci.2014;79:69-82.
[31] Zeng RC,Sun XX,Song YW,et al.Influence of solution temperature on corrosion resistance of Zn-Ca phosphate conversion coating on biomedical Mg-Li-Ca alloys.Trans Nonferrous Met Soc China.2013;23(11):3293−3299.
[32] González-Carrasco JL,Ciapetti G,Montealegre MA,et al. Evaluation of mechanical properties and biological response of an alumina-forming Ni-free ferritic alloy. Biomaterials. 2005;26:3861-3871.
[33] Xue W,Moore JL,Hosick HL,et al.Osteoprecursor cell response to strontium-containing hydroxyapatite ceramics.J Biomed Mater Res A.2006;79(4):804-814.
[34] Hui YY,MeAmis WC,Baynes JW,et al. Effect of advanced glycation end production oxidative stress in endothelial ceils in culture:a warning on the Use of cells studied in serum-free media.Diabetologia. 2001;44:1310-1317.
[35] Geiger B,Spatz JP,Bershadsky AD.Environmental sensing through focal adhesions.Nat Rev Mol Cell Biol.2009;10(1): 2l-33.
[36] Pegueroles M,Aparieio C,Bosio M,et al.Spatial organization of osteoblast fibroneetin matrix on titanium surfaces:effects of roughness,chemical heterogeneity and surface energy.Acta Biomater.2010;6(1):291-301. |