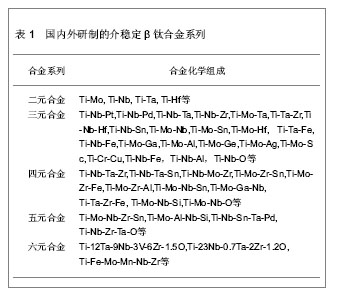
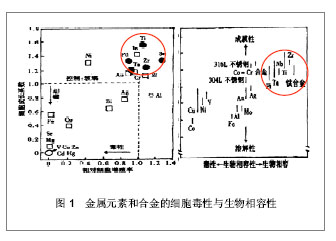
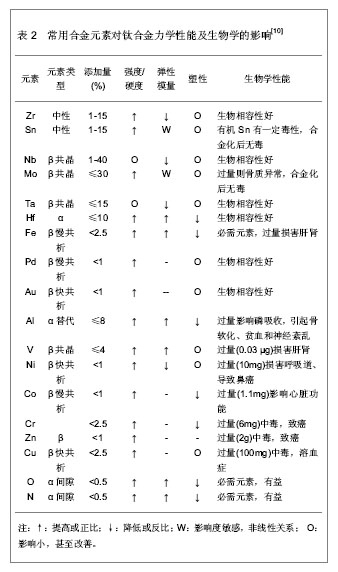
| [1] 于振涛,周廉,王克光.生物医用型型钛合金的设计与开发[J].稀有金属快报, 2004,23(1):5-10.[2] 于振涛,张明华,余森.中国医疗器械用钛合金材料研发、生产与应用现状分析[J].中国医疗器械信息,2012,18(7):1-8.[3] Long M,Rack HJ.Ti tanium alloys in total joint replacement—a materials science perspective. Biomaterials.1998;(19): 1621-1639.[4] 张玉梅.钛及钛合金在口腔科应用的研究方向[J].生物医学工程学杂志,2000,17(2): 206-208.[5] 于振涛, 余森, 张明华,等.外科植入物用新型医用钛合金材料设计、开发与应用现状及进展[J].中国材料进展,2010,29(12): 35-51.[6] Okazaki Y,Ito Y,Kyo K. Corrosion resistance and corrosion fatigue strength of new titanium alloys for medical implants without V and Al. Mater Sci Eng A.1996;213 (1-2):138-147.[7] Niinomi M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J Mech Behav Biomed Mater.2008; 1(1): 30-42.[8] Kuroda D,Niinnomi M,Morinaga M,et al. Design, mechanical properties of new b type titanium alloys for implant materials. Mater Sci Eng A.1998;A243: 244-249.[9] 郝玉琳,杨锐.纳米高强Ti-Nb-Zr-Sn合金[J].金属学报,2005, 41(11): 1183-1189.[10] 刘江龙.环境材料导论[M].北京:冶金工业出版社,1999.[11] Geetha M,Singh AK,Asokamani R,et al.Ti based biomaterials, the ultimate choice for orthopaedic implants-A review.Prog Mater Sci.2009;(54):397-425.[12] Karasevskaya OP,Ivasishin OM,Semiatin SL,et al. Deformation behavior of beta-titanium alloys.Mater Sci Eng A.2003;354(1-2): 121-132.[13] Grosdidier T,Philippe MJ.Deformation induced martensite and superelasticity in a β-metastable titanium alloy.Mater Sci Eng A.2000;291:218-223.[14] Saito T,Furuta T,Hwan JH,et al.Multifunctional alloys obtained via a dislocation-free plastic deformation mechanism. Science. 2003;300:464-467.[15] 刘瑞堂,刘文博,刘锦云.工程材料力学性能[M].哈尔滨:哈尔滨工业大学出版社,2001.[16] Valiev RZ,Islamgaliev RK,Alexandrov IV,et al.Bulk nanostructured materials from severe plastic deformation. Prog Mater Sci.2000; 45 (2): 103-189..[17] 于振涛,麻西群,余森,等.生物医用钛合金的微纳化加工技术及最新进展[J].中国有色金属学报,2010,20(s1):1008-1012.[18] Paital SR,Dahotre NB.Calcium phosphate coatings for bio-implant applications: Materials, performance factors, and methodologies. Mater Sci Eng R.2009; 66(1-3): 1-70.[19] Yu S,Yu Z,Wang G,et al.Biocompatibility and osteoconduction of active porous calcium-phosphate films on a novel Ti-3Zr-2Sn-3Mo-25Nb biomedical alloy. Colloids Surf B Biointerfaces. 2011; 85(2):83-115.[20] Yu ZT,Wang G, Ma XQ,et al. Development of Biomedical Near β Titanium Alloys. Mater Sci Forum.2009;(618-619): 303-306.[21] Webster TJ,Ergun C,Doremus RH,et al.Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000; 17(21): 1803-1810.[22] Grinevich A,Bacakova L,Choukourov A, et al. Nanocomposite Ti/hydrocarbon plasma polymer films from reactive magnetron sputtering as growth support for osteoblast-like and endothelial cells. J Biomed Mater Res A. 2009;88(4): 952–966.[23] 梁迎春,宋代平,陈明君,等,钛系生物医用材料表面粗糙度影响细胞黏附的新进展[J].机械工程学报,2008,44(7):6-10.[24] Zhou YL,Niinomi M,Akahori T .Effects of Ta content on Young’s modulus and tensile properties of binary Ti–Ta alloys for biomedical applications. Mater Sci Eng A.2004;371(25): 283-286.[25] Ryan G,Pandit A,Apatsidis DP. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials.2006; 27(13): 2651-2670.[26] Dohan Ehrenfest DM,Coelho PG,Kang BS,et al.Classification of osseointegrated implant surfaces: materials, chemistry and topography. Trends Biotechnol. 2010;28(4): 198-206. |
.jpg)