| [1] Redman SN, Oldfield SF, Archer CW. Current strategies for articular cartilage repair. Eur Cell Mater. 2005;9:23-32; discussion 23-32.[2] Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30(1):215-224.[3] Schnabel M, Marlovits S, Eckhoff G, et al. Dedifferentiation-associated changes in morphology and gene expression in primary human articular chondrocytes in cell culture. Osteoarthritis Cartilage. 2002;10(1):62-70.[4] Zhang Y, Cai G, Liu W, et al. Zhonghua Zhengxing Waike Zazhi. 2007;23(4):331-334.张艳,柴岗,刘伟,等.体外培养过程中去分化的人软骨细胞基因表达谱的变化[J].中华整形外科杂志,2007,23(4):331-334.[5] Karlsen TA, Shahdadfar A, Brinchmann JE. Human primary articular chondrocytes, chondroblasts-like cells, and dedifferentiated chondrocytes: differences in gene, microRNA, and protein expression and phenotype. Tissue Eng Part C Methods. 2011;17(2):219-227.[6] Barlic A, Drobnic M, Malicev E, et al. Quantitative analysis of gene expression in human articular chondrocytes assigned for autologous implantation. J Orthop Res. 2008;26(6): 847-853.[7] Wang L, Verbruggen G, Almqvist KF, et al. Flow cytometric analysis of the human articular chondrocyte phenotype in vitro. Osteoarthritis Cartilage. 2001;9(1):73-84.[8] Diaz-Romero J, Gaillard JP, Grogan SP, et al. Immunophenotypic analysis of human articular chondrocytes: changes in surface markers associated with cell expansion in monolayer culture. J Cell Physiol. 2005;202(3):731-742.[9] Chu CR, Szczodry M, Bruno S. Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev. 2010;16(1): 105-115.[10] Ren B, Li HF, Zuo XA, et al. Hebei Beifang Yixue Xuebao Yixue Ban. 2010;27(2):8-11.任保,李慧芳,左喜爱,等.兔关节软骨细胞的原代培养和去分化现象[J].河北北方医学学报:医学版,2010,27(2):8-11.[11] Xia Q, Wang WD, Wei Z, et al. Zhongguo Yishi Zazhi. 2007; 9(9):1174-1177.夏青,王卫东,魏振,等.兔关节软骨细胞的体外培养及鉴定[J].中国医师杂志,2007,9(9):1174-1177.[12] Kim YJ, Sah RL, Doong JY, et al. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174(1):168-176.[13] Enobakhare BO, Bader DL, Lee DA. Quantification of sulfated glycosaminoglycans in chondrocyte/alginate cultures, by use of 1,9-dimethylmethylene blue. Anal Biochem. 1996;243(1): 189-191.[14] Gosset M, Berenbaum F, Thirion S, et al. Primary culture and phenotyping of murine chondrocytes. Nat Protoc. 2008;3(8): 1253-1260.[15] Sasazaki Y, Seedhom BB, Shore R. Morphology of the bovine chondrocyte and of its cytoskeleton in isolation and in situ: are chondrocytes ubiquitously paired through the entire layer of articular cartilage? Rheumatology (Oxford). 2008;47(11): 1641-1646.[16] Giovannini S, Diaz-Romero J, Aigner T, et al. Population doublings and percentage of S100-positive cells as predictors of in vitro chondrogenicity of expanded human articular chondrocytes. J Cell Physiol. 2010;222(2):411-420.[17] Tew SR, Murdoch AD, Rauchenberg RP, et al. Cellular methods in cartilage research: primary human chondrocytes in culture and chondrogenesis in human bone marrow stem cells. Methods. 2008;45(1):2-9.[18] Li H. Shanxi Zhigong Yixueyuan Xuebao. 2008;18(1):15-17.李昊.不同年龄兔软骨细胞培养和形态学研究[J].山西职工医学院学报,2008,18(1):15-17.[19] Schulze-Tanzil G, de Souza P, Villegas Castrejon H, et al. Redifferentiation of dedifferentiated human chondrocytes in high-density cultures. Cell Tissue Res. 2002;308(3):371-379.[20] Martin I, Jakob M, Schafer D, et al. Quantitative analysis of gene expression in human articular cartilage from normal and osteoarthritic joints. Osteoarthritis Cartilage. 2001;9(2): 112-118.[21] Diaz-Romero J, Nesic D, Grogan SP, et al. Immunophenotypic changes of human articular chondrocytes during monolayer culture reflect bona fide dedifferentiation rather than amplification of progenitor cells. J Cell Physiol. 2008;214(1):75-83.[22] The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30. |
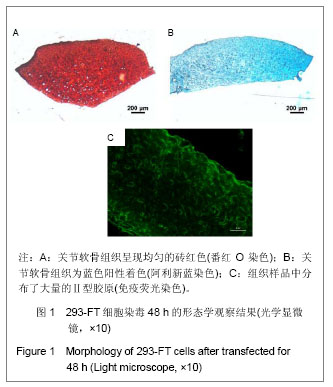
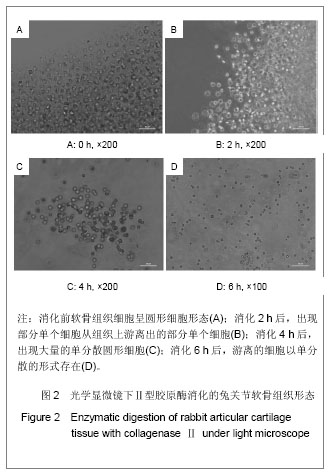
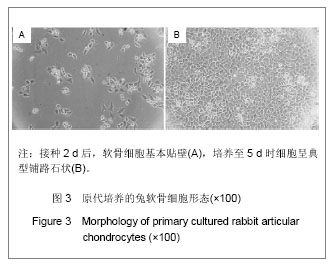
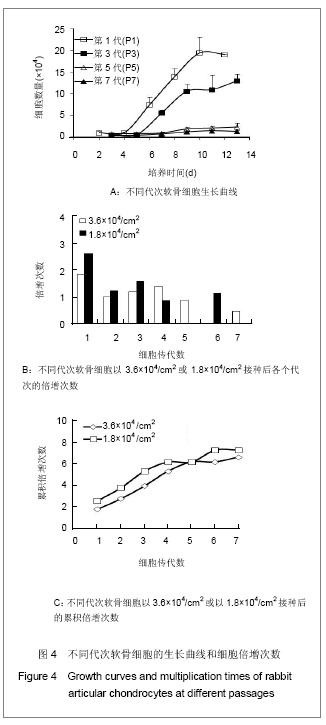
.jpg)
.jpg)