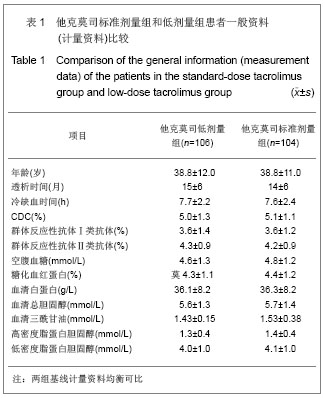
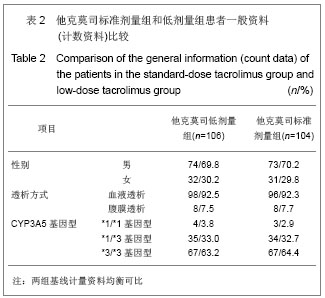
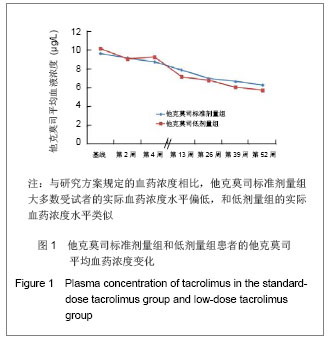
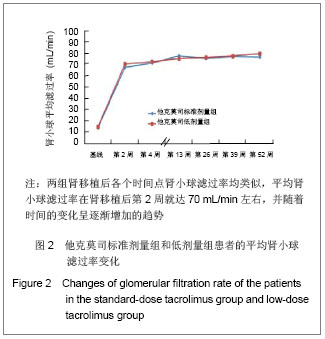
| [1] Li L, Sun Q. Renal transplantation in China: ten years of experience at Nanjing Jinling Hospital. Clin Transpl. 2006: 71-77.[2] Lodhi SA, Meier-Kriesche HU. Kidney allograft survival: the long and short of it. Nephrol Dial Transplant. 2011;26(1):15-17.[3] Morales JM, Marcén R, Del Castillo D, et al. Risk factors for graft loss and mortality after renal transplantation according to recipient age: a prospective multicentre study.Nephrol Dial Transplant. 2012;27 Suppl 4:iv39-iv46.[4] Phelan PJ, O'Kelly P, Tarazi M,et al. Renal allograft loss in the first post-operative month: causes and consequences.Clin Transplant. 2012;26(4):544-549.[5] Ekberg H, Bernasconi C, Nöldeke J, et al. Cyclosporine, tacrolimus and sirolimus retain their distinct toxicity profiles despite low doses in the Symphony study. Nephrol Dial Transplant. 2010;25(6):2004-2010.[6] Arend SM, Mallat MJ, Westendorp RJ,et al. Patient survival after renal transplantation; more than 25 years follow-up. Nephrol Dial Transplant. 1997;12(8):1672-1679.[7] Ekberg H, Tedesco-Silva H, Demirbas A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357(25):2562-2575.[8] Matas AJ. Chronic progressive calcineurin nephrotoxicity: an overstated concept. Am J Transplant. 2011;11(4):687-692.[9] Tan TC,Robinson PJ.Mechanisms of calcineurin inhibitor-induced neurotoxicity.Transplantation Reviews.2006; 20(1):49-60.[10] Djamali A, Reese S, Hafez O, et al. Nox2 is a mediator of chronic CsA nephrotoxicity.Am J Transplant. 2012;12(8): 1997-2007.[11] Matas AJ.Chronic progressive calcineurin nephrotoxicity: an overstated concept.Am J Transplant. 2011;11(4):687-692.[12] Pascual M, Theruvath T, Kawai T, et al. Strategies to improve long-term outcomes after renal transplantation. N Engl J Med. 2002;346(8):580-590.[13] Burkhalter F, Oettl T, Descoeudres B, et al. High incidence of rejection episodes and poor tolerance of sirolimus in a protocol with early steroid withdrawal and calcineurin inhibitor-free maintenance therapy in renal transplantation: experiences of a randomized prospective single-center study. Transplant Proc. 2012;44(10):2961-2965.[14] Roberts DM, Jiang SH, Chadban SJ.The treatment of acute antibody-mediated rejection in kidney transplant recipients-a systematic review.Transplantation. 2012;94(8):775-783.[15] Isoniemi HM, Krogerus L, von Willebrand E,et al. Histopathological findings in well-functioning, long-term renal allografts. Kidney Int. 1992;41(1):155-160.[16] Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31-41.[17] Poggio ED, Wang X, Greene T, et al. Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol. 2005;16(2):459-466.[18] Qi J,Min ZL,Chang JW,et al. Zhonghua Qiguan Yizhi Zazhi. 2000;21(5):288-291.齐隽,闵志廉,常继伟,等.肾移植后免疫抑制用药方案与移植肾长期存活的关系[J].中华器官移植杂志,2000,21(5):288-291.[19] Xu D,Liu Y,Wang XH,et al. Zhonghua Qiguan Yizhi Zazhi. 2000; 21(5):285-287.徐达,刘永,王祥慧,等.用于肾移植的几种免疫抑制方案的对比研究[J]. 中华器官移植杂志,2000,21(5):285-287.[20] Morris RE.The future of immunosuppression: a personal view.Transplant Proc. 2004;36(2 Suppl):577S-579S.[21] Hardinger KL, Schnitzler MA, Koch MJ, et al. Cyclosporine minimization and cost reduction in renal transplant recipients receiving a C2-monitored, cyclosporine-based quadruple immunosuppressive regimen.Transplantation. 2004;78(8): 1198-1203.[22] Pang GX,Lu GC. Zhongguo Yaofang. 2008;19(16):1211-1213.庞国勋,陆国椿.我国2004-2006年免疫抑制剂市场状况分析[J].中国药房, 2008,19(16):1211-1213.[23] Nankivell BJ, Wavamunno MD, Borrows RJ,et al. Mycophenolate mofetil is associated with altered expression of chronic renal transplant histology.Am J Transplant. 2007; 7(2):366-376.[24] Boudjema K, Camus C, Saliba F, et al. Reduced-dose tacrolimus with mycophenolate mofetil vs. standard-dose tacrolimus in liver transplantation: a randomized study. Am J Transplant. 2011;11(5):965-976.[25] Cortesini R.Cyclosporine--lessons from the first 20 years. Transplant Proc. 2004;36(2 Suppl):158S-162S.[26] Burdmann EA, Andoh TF, Yu L, et al. Cyclosporine nephrotoxicity. Semin Nephrol. 2003;23(5):465-476.[27] Bakker RC,Scholten EM,de Fijter JW,et al. Chronic cyclosporine nephrotoxicity in renal transplantation. Transplantation Reviews.2004;18(1):54-64.[28] Maes BD, Vanrenterghem YF.Cyclosporine: advantages versus disadvantages vis-à-vis tacrolimus.Transplant Proc. 2004;36(2 Suppl):40S-49S.[29] Lucan M, Iacob G, Lucan C, et al.Ten years of cyclosporine use in renal transplantation: a single-center experience with 479 renal transplants.Transplant Proc. 2004;36(2 Suppl): 177S-180S.[30] Weimer R, Deisz S, Dietrich H, et al. Impact of maintenance immunosuppressive regimens--balance between graft protective suppression of immune functions and a near physiological immune response.Transpl Int. 2011;24(6): 596-609. [31] Naesens M, Lerut E, Sarwal M, et al. Balancing efficacy and toxicity of kidney transplant immunosuppression.Transplant Proc. 2009;41(8):3393-3395.[32] Jørgensen KA, Povlsen JV, Poulsen JH. Optimal time for determination of blood tacrolimus level.Transplant Proc. 2001;33(7-8):3164-3165.[33] Ji SM,Chen JS,Sun QQ,et al. Yixue Yanjiusheng Xuebao. 2011;24(1):34-38.季曙明,陈劲松,孙启全,等.环孢素转换至他克莫司治疗慢性移植肾肾病的临床和病理观察[J].医学研究生学报,2011,24(1): 34-38.[34] Zhu YH,Min ZL,Wang LM,et al. Zhonghua Qiguan Yizhi Zazhi. 2000;21(2):119-121.朱有华,闵志廉,王立明,等.普乐可复在肾移植中的临床应用[J].中华器官移植杂志, 2000,21(2):119-121.[35] Roberts DM, Jiang SH, Chadban SJ.The treatment of acute antibody-mediated rejection in kidney transplant recipients-a systematic review.Transplantation. 2012;94(8):775-783.[36] Ji SM, Liu ZH, Chen JS, et al. Rescue therapy by immunoadsorption in combination with tacrolimus and mycophenolate mofetil for C4d-positive acute humoral renal allograft rejection.Transplant Proc. 2006;38(10):3459-3463.[37] Ji SM, Wen JQ, Cheng DR, et al.Conversion from Calcineurin Inhibitors to Sirolimus Maintenance Therapy in Renal Allograft Recipients with Risk Factors. Open Journal of Organ Transplant Surgery. 2011;1(1): 8-13. [38] Ji SM, Liu ZH, Wu D,et al.Surveillance Renal Allograft Biopsy on Diagnosis of BK Virus Nephropathy in Chinese Renal Transplant Recipients. Open Journal of Organ Transplant Surgery. 2012;2(4):62-68.[39] John R, Herzenberg AM. Our approach to a renal transplant biopsy. J Clin Pathol. 2010;63(1):26-37.[40] Ji SM,Wen JQ,Cheng DR,et al. Qiguan Yizhi. 2012;3(2): 95-101.季曙明,文吉秋,程东瑞,等.采用程序化肾活检评估肾移植后低水平和标准水平他克莫司的疗效[J].器官移植,2012,3(2):95-101.[41] Matas AJ. Chronic progressive calcineurin nephrotoxicity: an overstated concept. Am J Transplant. 2011;11(4):687-692.[42] Ji SM, Li LS, Sun QQ,et al. Immunoregulation of thymosin alpha 1 treatment of cytomegalovirus infection accompanied with acute respiratory distress syndrome after renal transplantation.Transplant Proc. 2007;39(1):115-119.[43] Zu Q,Yao B,Yu LX. Shenzangbing yu Touxi Shenyizhi Zazhi. 2000;9(3):251-253.祖强,姚冰,于立新.FK506的肾毒性[J].肾脏病与透析肾移植杂志,2000,9(3):251-253.[44] Kidokoro K, Satoh M, Nagasu H,et al.Tacrolimus Induces Glomerular Injury via Endothelial Dysfunction Caused by Reactive Oxygen Species and Inflammatory Change.Kidney Blood Press Res. 2012;35(6):549-557.[45] Gijsen VM, Madadi P, Dube MP, et al. Tacrolimus-induced nephrotoxicity and genetic variability: a review.Ann Transplant. 2012;17(2):111-121.[46] Horike K, Takeda A, Yamaguchi Y, et al. Is arteriolar vacuolization a predictor of calcineurin inhibitor nephrotoxicity. Clin Transplant. 2011;25 Suppl 23:23-27.[47] Pirsch JD, Miller J, Deierhoi MH, et al. A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation. FK506 Kidney Transplant Study Group.Transplantation. 1997;63(7):977-983.[48] Weir MR, Ward MT, Blahut SA, et al. Long-term impact of discontinued or reduced calcineurin inhibitor in patients with chronic allograft nephropathy. Kidney Int. 2001;59(4): 1567-1573.[49] Duijnhoven EM, Boots JM, Christiaans MH, et al. Influence of tacrolimus on glucose metabolism before and after renal transplantation: a prospective study. J Am Soc Nephrol. 2001; 12(3):583-588.[50] First MR, Gerber DA, Hariharan S,et al. Posttransplant diabetes mellitus in kidney allograft recipients: incidence, risk factors, and management.Transplantation. 2002;73(3): 379-386.[51] Campistol JM. Long-term maintenance therapy with calcineurin inhibitors: an update.Transplant Proc. 2010;42 (9 Suppl):S21-24.[52] Undre NA, Stevenson P, Schäfer A. Pharmacokinetics of tacrolimus: clinically relevant aspects.Transplant Proc. 1999; 31(7A):21S-24S.[53] Marcén R. Immunosuppressive drugs in kidney transplantation: impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs. 2009;69(16):2227-2243. [54] Meier-Kriesche HU, Merville P, Tedesco-Silva H,et al. Mycophenolate mofetil initiation in renal transplant patients at different times posttransplantation: the TranCept Switch study. Transplantation. 2011;91(9):984-990.[55] Sollinger HW. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. U.S. Renal Transplant Mycophenolate Mofetil Study Group. Transplantation. 1995;60(3):225-232.[56] European Mycophenolate Mofetil Cooperative Study Group. Placebo-controlled study of mycophenolate mofetil combined with cyclosporin and corticosteroids for prevention of acute rejection. Lancet. 1995;345(8961):1321-1325.[57] The Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group. A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. Transplantation. 1996;61(7):1029-1037. [58] Stegall MD, Park WD, Larson TS,et al. The histology of solitary renal allografts at 1 and 5 years after transplantation. Am J Transplant. 2011;11(4):698-707. |