| [1] Yan T,Ao NJ,Tan BH,et al.Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2008;12(10):1904-1905. 严拓,敖宁建,覃百花,等.人工角膜及其相关材料[J].中国组织工程研究与临床康复,2008,12(10):1904-1905.[2] Chen JJ,Huang ZP.Guoji Yanke Zazhi. 2008;8(3):561-564. 陈佳佳,黄振平.人工角膜研究进展[J].国际眼科杂志,2008,8(3): 561-564.[3] Li N, Zhou W, Sun H, Yixue Zongshu. 2009;15(4):575-578. 李娜,周伟,孙恒.人工角膜的研究进展[J].医学综述,2009,15(4): 575-578.[4] Zhang X,Li XY,ZhongYY MZ,et al.Zhongguo Shiyong Yanke Zazhi. 2010;28(12):1279-1284. 张旭,李效岩,中原由木子,等.人工角膜研究进展[J].中国实用眼科杂志,2010,28(12):1279-1284.[5] Xu FL.Sichuan:Sichuan Daxue.2005. 许凤兰.纳米羟基磷灰石/水凝胶人工角膜研究[D].四川:四川大学,2005.[6] Xu FL,Li YB,Yao XM,et al.Fuhe Cailiao Xuebao. 2005;22(1): 27-31. 许凤兰,李玉宝,姚晓明,等.纳米羟基磷灰石/聚乙烯醇复合人工角膜材料[J].复合材料学报,2005,22(1):27-31.[7] Yao XM,Deng HW,Li YB,et al.Zhongguo Shiyong Yanke Zazhi. 2004;22(8):667-669. 姚晓明,邓宏伟,李玉宝,等.多孔纳米羟基磷灰石-聚乙烯醇水凝胶人工角膜的实验研究[J].中国实用眼科杂志,2004,22(8): 667-669.[8] Jia SC. Sichuan:Sichuan Daxue. 2007. 贾舜宸.原位聚合法制备DL聚乳酸、β-磷酸三钙复合骨修复材料及性能研究[D].四川:四川大学,2007.[9] Gong L.Chongqing:Chongqing Daxue.2007. 龚立.多孔β-磷酸三钙/明胶复合支架的制备及性能研究[D].重庆:重庆大学,2007.[10] Zhang SH.Anhui:Hefei Gongye Daxue.2002. 张士华.β-磷酸三钙粉末和多孔β-磷酸三钙生物陶瓷的研制[D].安徽:合肥工业大学,2002.[11] Guo DG,Xu KW.Shengwu Yixue Gongchengcue Zazhi. 2005; 22(3):602-605. 郭大刚,徐可为.聚乙烯醇在生物医学工程中的应用研究进展[J].生物医学工程杂志,2005,22(3):602-605.[12] Liu K,Li Y,Xu F,et al.Graphite/poly(vinyl alcohol) hydrogel composite as porous ringy skirt for artificial cornea. Mat Sci Eng.2009;29(1):261-266.[13] FangJY,Lin ZM,Ling JQ,et al.Zhonghua Kouqiang Yixue Yanjiu Zazhi. 2010;4(6):540-547. 房俊艳,林正梅,凌均棨,等.低收缩性树脂封闭剂的研制及其生物相容性研究[J]. 中华口腔医学研究杂志,2010,4(6):540-547.[14] Zhou Y,Cheng GG,Yu XG,et al. Zhongguo Zuzhi Gongcheng Yanjiu. 2012;16(8):1401-1406. 周岩,程钢戈,余新光,等.具有生物活性的高导复合支架材料制备表征及其生物相容性[J]. 中国组织工程研究,2012,16(8): 1401-1406.[15] Su B,Jiang DM,Yan YG,et al.Disan Junyi Daxue Xuebao. 2011;33(1):40-44. 苏保,蒋电明,严永刚,等.氨基酸聚合物/硫酸钙生物工程骨的生物相容性研究[J]. 第三军医大学学报,2011,33(1):40-44.[16] Li R,Wang QS. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2011;15(29):5471-5474. 李瑞,王青山.生物材料生物相容性的评价方法和发展趋势[J].中国组织工程研究与临床康复,2011,15(29):5471-5474.[17] GB/T 16886.10-2005医疗器械生物学评价,第10部分:刺激与迟发型超敏反应试验[S].2005:1-39.[18] YY/T 127.2-2009口腔医疗器械生物学评价,第2单元:试验方法急性全身毒性试验:静脉途径.[S]. 2009:1-2.[19] GB/T1 6886.5-2003医疗器械生物学评价,第5部分:体外细胞毒性试验[S].81-88.[20] Yan JH,Sun HM,Shang HW,et al.Jilin Nongye Daxue Xuebao. 2011;33(5): 522-526. 闫继红,孙海梅,尚宏伟,等.可注射性壳聚糖基温敏性凝胶的制备及其生物相容性[J]. 吉林农业大学学报,2011,33(5): 522-526.[21] Ye X,Zhu L,Tang GX,et al.Zhonghua Shenjing Waike Jibing Yanjiu Zazhi. 2011;10(6):543-547. 叶迅,朱琳,唐光昕,等.纯钛表面梯度生物活性涂层材料生物相容性研究[J].中华神经外科疾病研究杂志,2011,10(6):543-547.[22] Shen LJ. Hunan:Hunan Daxue.2011. 申林静.生物材料的生物相容性研究[D].湖南:湖南大学,2011.[23] Deng JH.Guangxi:Guangxi Yike Daxue.2011. 邓建华.京尼平生物型人工食管生物相容性的实验评价[D].广西:广西医科大学,2011[24] Wang LX.Hunan:Hunan Daxue.2010. 汪兰香.二茂铁羧酸、醇、酯的合成及细胞毒活性研究[D].湖南:湖南大学,2010.[25] Zhang YB,Jiang M,Hu P,et al.Zhonghua Shiyong Zhenduan yu Zhiliao Zazhi. 2011;25(10):944-946. 张燕搏,姜明,胡平,等.聚氨酯不同低温等离子体改性及生物相容性评价[J].中华实用诊断与治疗杂志,2011,25(10):944-946.[26] 国家食品药品监督管理局.YBB0006-2003皮内刺激检查法[S].2003:18-19.[27] GB/T 16886.1-2001医疗器械生物学评价,第1部分:评价与试验[S]2001:21-33.[28] Guan J,Gao P,Huang MK,et al. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2009;13(12):2227-2231. 关静,高萍,黄妹杰,等.聚乙烯醇水凝胶的生物相容性评价[J].中国组织工程研究与临床康复,2009,13(12):2227-2231. |

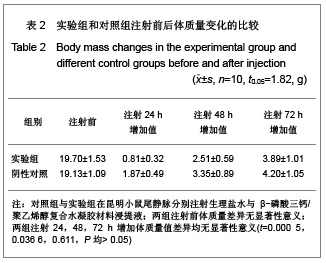
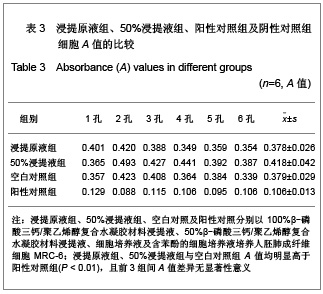
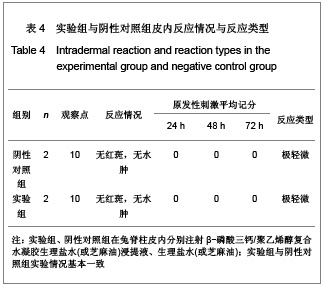
.jpg)
.jpg)
.jpg)