| [1] Meirelles Júnior RF, Salvalaggio P, Rezende MB, et al. Liver transplantation: history, outcomes and perspectives. Einstein (Sao Paulo). 2015;13(1): 149-152.[2] 丁易涛,施晓雷.生物人工肝脏临床研究现状[J].中国研究型医院, 2017,4(5): 17-25.[3] Sakiyama R, Blau BJ, Miki T. Clinical translation of bioartificial liver support systems with human pluripotent stem cell-derived hepatic cells. World J Gastroenterol. 2017;23(11):1974-1979.[4] Forbes SJ, Gupta S, Dhawan A. Cell therapy for liver disease: From liver transplantation to cell factory. J Hepatol. 2015;62(1 Suppl):S157-169.[5] Kim Y, Kang K, Yoon S, et al. Prolongation of liver-specific function for primary hepatocytes maintenance in 3D printed architectures. Organogenesis. 2018;14(1):1-12.[6] Lancett P, Williamson B, Barton P, et al. Development and Characterization of a Human Hepatocyte Low Intrinsic Clearance Assay for Use in Drug Discovery. Drug Metab Dispos. 2018;46(8):1169-1178.[7] Fang ZP, Jiang BG, Gu XF, et al. P21-activated kinase 5 plays essential roles in the proliferation and tumorigenicity of human hepatocellular carcinoma. Acta Pharmacol Sin. 2014;35(1):82-88.[8] van Wenum M, Treskes P, Tang CY, et al. Scaling-up of a HepaRG progenitor cell based bioartificial liver: optimization for clinical application and transport. Biofabrication. 2017;9(3):035001. [9] Layton DS, Bean AG, Dodge NM, et al. Differential cytokine expression and regulation of human anti-pig xenogeneic responses by modified porcine dendritic cells. Xenotransplantation. 2008;15(4):257-267.[10] 石伟,蔡端,张群华,等. 异种肝细胞移植缓解大鼠急性肝功能衰竭及其排斥反应观察[J]. 中华肝胆外科杂志, 2005,11(4):265-268.[11] Yang G, Hong H, Torres A, et al. Standards for Deriving Nonhuman Primate-Induced Pluripotent Stem Cells, Neural Stem Cells and Dopaminergic Lineage. Int J Mol Sci. 2018;19(9): E2788.[12] Steens J, Klein D. Current Strategies to Generate Human Mesenchymal Stem Cells In Vitro. Stem Cells Int. 2018;2018:6726185.[13] Hansel MC, Gramignoli R, Blake W, et al. Increased reprogramming of human fetal hepatocytes compared with adult hepatocytes in feeder-free conditions. Cell Transplant. 2014;23(1):27-38.[14] Crombleholme TM, Langer JC, Harrison MR, et al. Transplantation of fetal cells. Am J Obstet Gynecol. 1991;164(1 Pt 1):218-230.[15] Zhang Y, Shi J, Liu S. Establishment and Characterization of a Telomerase-Immortalized Sheep Trophoblast Cell Line. Biomed Res Int. 2016;2016:5808575.[16] 卢扬洲,黎少,姜华,等. 新型永生化人肝细胞系HepZJ的安全性研究[J]. 药物评价研究,2018,41(6):1062-1067.[17] Ra JC, Shin IS, Kim SH, et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;20(8):1297-1308.[18] 王琼,汤钱球,张小云,等.炎症和凝血指标在脓毒血症诊断和预后评估中价值分析[J].临床血液学杂志(输血与检验), 2018,31(5):750-753.[19] Thorell SE, Nash MJ, Thachil J. Clinical implications of clotting screens. Int J Lab Hematol. 2015;37(1):8-13.[20] Eschbach D, Horst K, Sassen M, et al. Hypothermia does not influence liver damage and function in a porcine polytrauma model. Technol Health Care. 2018;26(2):209-221.[21] Long C, Yang J, Yang H, et al. Attenuation of renal ischemia/reperfusion injury by oleanolic acid preconditioning via its antioxidant, anti?inflammatory, and anti?apoptotic activities. Mol Med Rep. 2016;13(6): 4697-4704.[22] 国家食品药品监督管理局药品审评中心. 治疗用生物制品非临床安全性评价指导原则[EB/OL]. http://www.cde.org.cn/zdyz.do?method= largePage&id=100, 2010-05-06/2018-10-29.[23] Stacey GN, Coecke S, Price AB, et al. Ensuring the Quality of Stem Cell-Derived In Vitro Models for Toxicity Testing. Adv Exp Med Biol. 2016; 856:259-297.[24] Van Meer PJ, Graham ML, Schuurman HJ. The safety, efficacy and regulatory triangle in drug development: Impact for animal models and the use of animals. Eur J Pharmacol. 2015;759:3-13.[25] Misik J, Pavlikova R, Cabal J, et al. Acute toxicity of some nerve agents and pesticides in rats. Drug Chem Toxicol. 2015;38(1):32-36.[26] Christapher PV, Parasuraman S, Asmawi MZ, et al. Acute and subchronic toxicity studies of methanol extract of Polygonum minus leaves in Sprague Dawley rats. Regul Toxicol Pharmacol. 2017;86:33-41. [27] Xu S, Zhang Z, Chu M. Long-term toxicity of reduced graphene oxide nanosheets: Effects on female mouse reproductive ability and offspring development. Biomaterials. 2015;54:188-200.[28] Kuroda T, Yasuda S, Sato Y. Tumorigenicity studies for human pluripotent stem cell-derived products. Biol Pharm Bull. 2013;36(2):189-192.[29] De Matteis V. Exposure to Inorganic Nanoparticles: Routes of Entry, Immune Response, Biodistribution and In Vitro/In Vivo Toxicity Evaluation.Toxics. 2017;5(4): E29.[30] 曾颖星,郑永霞,徐艳雯,等. 肝细胞移植治疗肝病的研究进展[J]. 医学综述, 2018, 24(7): 1344-1348.[31] You S, Zhu B, Liu H, et al. Safety of Human Hepatoma Cell-Line Constructing Bioartificial Liver Supporting System Treating Patients with Liver Failure. Hepatogastroenterology. 2014;61(132):933-936.[32] Yun JW, Ahn JH, Kwon E, et al. Human umbilical cord-derived mesenchymal stem cells in acute liver injury: Hepatoprotective efficacy, subchronic toxicity, tumorigenicity, and biodistribution. Regul Toxicol Pharmacol. 2016;81:437-447.[33] 国家食品药品监督管理总局药品审评中心.人体细胞治疗研究和制剂质量控制技术指导原则[EB/OL]. http://www.cde.org.cn/zdyz.do?method= largePage&id=38, 2008-09-04/2018-10-29.[34] Food and Drug Administration, HHS. International Conference on Harmonisation; addendum to International Conference on Harmonisation Guidance on S6 Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals; availability. Notice. Fed Regist. 2012;77(97): 29665-29666.[35] 国家食品药品监督管理总局药品审评中心.细胞制品研究与评价技术指导原则(征求意见稿)[EB/OL]. http://www.cde.org.cn/news.do?method= largeInfo&id=313749, 2016-12-19/2018-10-29.[36] 国家食品药品监督管理总局.《药物非临床研究质量管理规范》(国家食品药品监督管理总局令第34号)[EB/OL]. http://www.gov.cn/gongbao/content/ 2017/content_5241929.htm, 2017-08-02/2018-10-29.[37] He J, Ruan GP, Yao X, et al. Chronic Toxicity Test in Cynomolgus Monkeys For 98 Days with Repeated Intravenous Infusion of Cynomolgus Umbilical Cord Mesenchymal Stem Cells. Cell Physiol Biochem. 2017;43(3):891-904.[38] Mäkelä T, Takalo R, Arvola O, et al. Safety and biodistribution study of bone marrow-derived mesenchymal stromal cells and mononuclear cells and the impact of the administration route in an intact porcine model. Cytotherapy. 2015;17(4):392-402.[39] Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54-63.[40] 汪溪洁,马璟. 药物安全性评价新技术和新方法研究进展[J]. 中国医药工业杂志,2017,48(3):341-350.[41] Kandhare AD, Bodhankar SL, Mohan V, et al. Acute and repeated doses (28 days) oral toxicity study of Vicenin-1, a flavonoid glycoside isolated from fenugreek seeds in laboratory mice. Regul Toxicol Pharmacol. 2016; 81:522-531. |
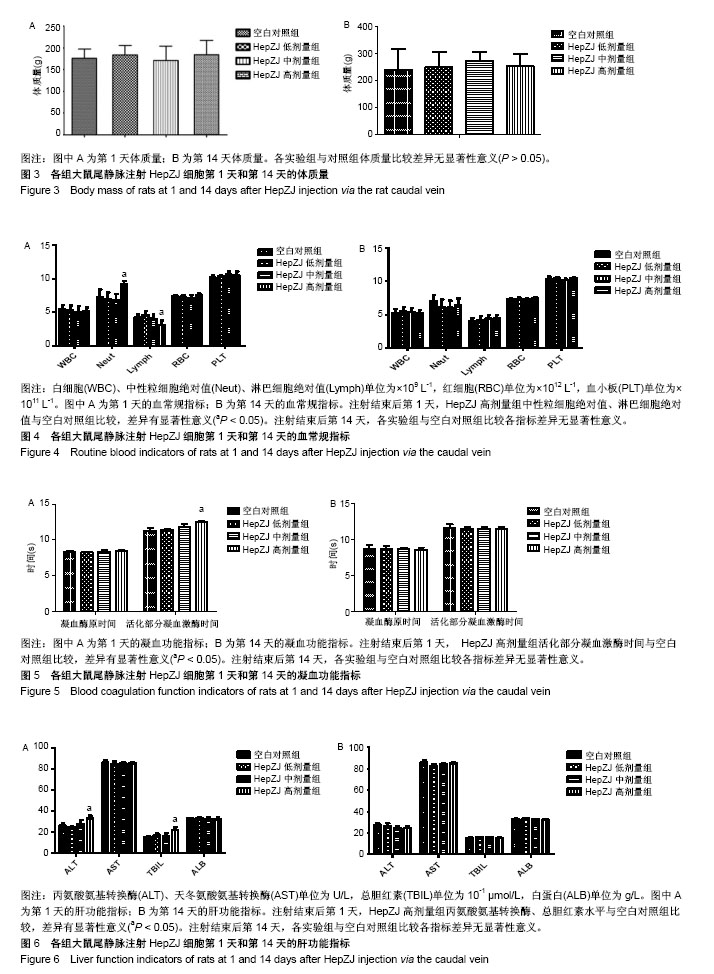
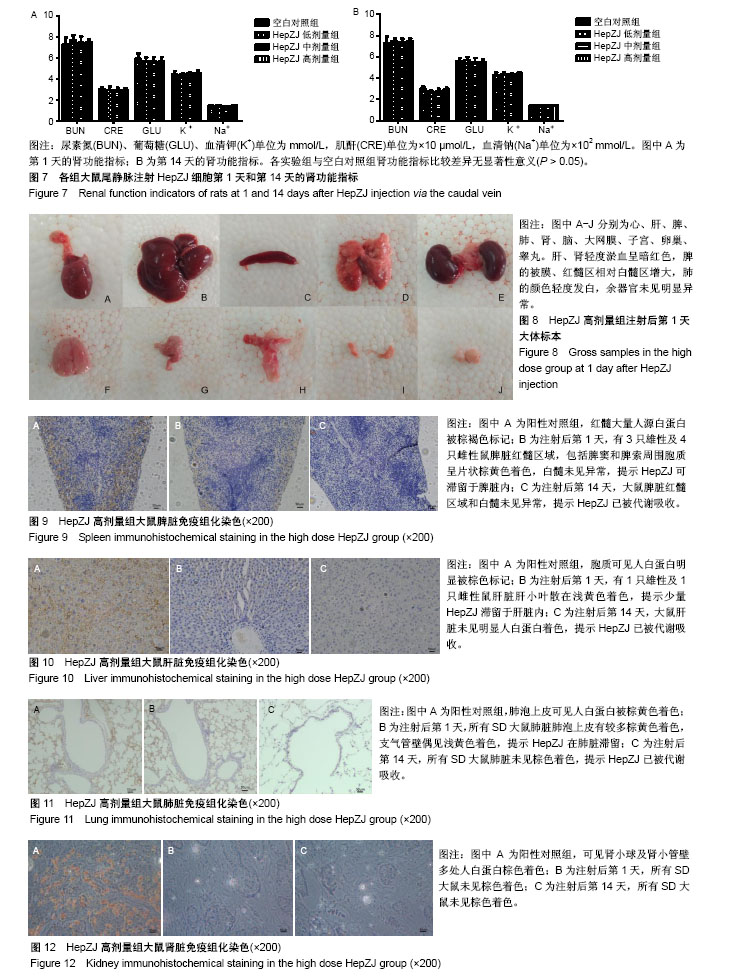
.jpg)
.jpg)
.jpg)
.jpg)