中国组织工程研究 ›› 2019, Vol. 23 ›› Issue (1): 110-117.doi: 10.3969/j.issn.2095-4344.1531
• 干细胞综述 stem cell review • 上一篇 下一篇
干细胞心肌向分化成熟过程中的物理刺激方法
张泽茜1,2,伍佳琪1,2,樊瑜波1,2,郑丽沙1,2
- 1北京航空航天大学生物与医学工程学院,生物力学与力生物学教育部重点实验室,北京市 100083;2北京航空航天大学生物医学工程高精尖创新中心,北京市 100083
-
修回日期:2018-09-22出版日期:2019-01-08发布日期:2018-11-28 -
通讯作者:郑丽沙,博士,副教授,硕士生导师,北京航空航天大学生物与医学工程学院,生物力学与力生物学教育部重点实验室,北京市 100083;北京航空航天大学生物医学工程高精尖创新中心,北京市 100083 -
作者简介:张泽茜,女,1997年生,黑龙江省哈尔滨市人,汉族,在读本科生,主要从事力学生物学及组织工程基础研究。 并列第一作者:伍佳琪,女,1997年生,湖南省常德市人,汉族,在读本科生,主要从事力学生物学及组织工程基础研究。 -
基金资助:科技部国家重点研发计划(2017YFC0108505),项目负责人:郑丽沙;国家自然科学基金(11572030),项目负责人:郑丽沙;国家自然科学基金(11120101001,11421202,11827803),项目负责人:樊瑜波;中央高校基本科研业务费专项经费(111计划)(B13003),项目负责人:郑丽沙
Physical stimulation methods promote myocardial differentiation and maturation of stem cells
Zhang Zeqian1, 2, Wu Jiaqi1, 2, Fan Yubo1, 2, Zheng Lisha1, 2
- 1School of Biological Science and Medical Engineering, Beihang University, Beijing 100083, China; 2Beijing Advanced Innovation Center for Biomedical Engineering, Beihang University, Beijing 100083, China
-
Revised:2018-09-22Online:2019-01-08Published:2018-11-28 -
Contact:Zheng Lisha, PhD, Associate professor, Master’s supervisor, School of Biological Science and Medical Engineering, Beihang University, Beijing 100083, China; Beijing Advanced Innovation Center for Biomedical Engineering, Beihang University, Beijing 100083, China -
About author:Zhang Zeqian, School of Biological Science and Medical Engineering, Beihang University, Beijing 100083, China; Beijing Advanced Innovation Center for Biomedical Engineering, Beihang University, Beijing 100083, China. Wu Jiaqi, School of Biological Science and Medical Engineering, Beihang University, Beijing 100083, China; Beijing Advanced Innovation Center for Biomedical Engineering, Beihang University, Beijing 100083, China. Zhang Zeqian and Wu Jiaqi contributed equally to this work. -
Supported by:the National Key Research and Development Program of the Ministry of Science and Technology of China, No. 2017YFC0108505 (to ZLS); the National Natural Science Foundation of China, No. 11572030 (to ZLS), 11120101001 (to FYB), 11421202 (to FYB), 11827803 (to FYB); the Fundamental Research Funds for the Central Universities (111 Plan), No. B13003 (to ZLS)
摘要:
文章快速阅读:
.jpg)
文题释义: 物理因素:干细胞生长的物理微环境对细胞形状、大小、排列方向、分化方向有重要影响,可以通过加载机械负荷、施加电刺激、调节基质硬度和拓扑结构、三维培养等物理方法来模拟心肌细胞生长的微环境,促进干细胞源心肌细胞的成熟。 干细胞心肌向分化:常见的干细胞如胚胎干细胞、诱导多能干细胞、间充质干细胞、心脏干细胞等具有心肌向分化潜能。这些干细胞产生的心肌细胞有明确的心脏表型,在体外和体内都能明显增殖,可以用于建立心脏药理模型以及心肌细胞治疗。
中图分类号:
引用本文
张泽茜,伍佳琪,樊瑜波,郑丽沙. 干细胞心肌向分化成熟过程中的物理刺激方法[J]. 中国组织工程研究, 2019, 23(1): 110-117.
Zhang Zeqian, Wu Jiaqi, Fan Yubo, Zheng Lisha. Physical stimulation methods promote myocardial differentiation and maturation of stem cells[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(1): 110-117.
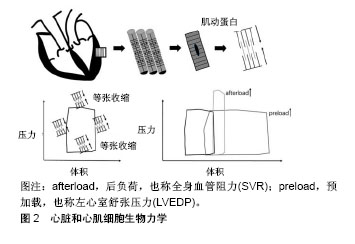
2.1.1 生物学基础 心脏的最小功能单位是肌节。在心脏中,肌节定向排列,被组装成连续的肌肉,这些肌肉的协调收缩使得心脏不断搏动。每个收缩周期可显示为压力-容积环(通常通过电导导管测量),包括等渗肌节收缩和等渗舒张。如果病理性增加全身血管阻力(例如二尖瓣关闭不全)或左心室舒张压力(例如主动脉瓣狭窄),这些阶段会缩短或延长,心脏和心肌细胞生物力学见图2。心肌细胞一直承受着血液流动过程中产生的压力、流动剪切力(摩擦力)等力学刺激[16]。研究表明,心肌细胞收缩时产生的血流动力学可以促进心脏发育,并在细胞水平上诱导心肌基因的表达和分化,促进心肌成熟[17]。
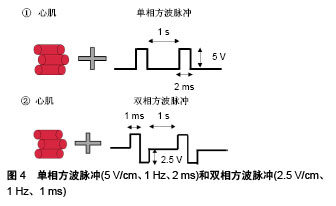
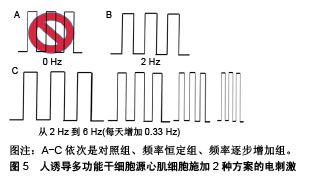
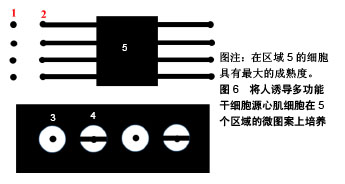
2.3.2 基底表面形貌 成人心肌是各向异性、互相平行排列的,有许多研究通过构建空间图案来模拟心肌生长微环境的微尺度拓扑形貌[61]。常用的技术有拓扑结构表面图案化和化学表面图案化[62]。拓扑结构表面图案化旨在改变基底的表面纹理,如通过微加工技术制作有序排列的突起或凹陷即沟槽结构,可以调节细胞骨架的定向排列和细胞形状。例如,有研究将大鼠心肌细胞接种在宽度450 nm、深度为100 nm或350 nm的聚氨酯凹槽上,心肌细胞的取向和伸长随着凹槽深度的增加而增加[63]。这可能是由于深度增加时,深宽比也增加,细胞的丝状伪足不能渗入沟槽并感应到沟槽的底部而被迫锚定在脊上,导致细胞沿凹槽和脊的方向对齐[61]。Carson等[64]研究了基质凹槽宽度对心肌成熟的影响,他们将人诱导多功能干细胞源心肌细胞接种到宽度范围350-2 000 nm的聚氨酯丙烯酸酯凹槽上,在700-1 000 nm宽度范围凹槽上的细胞有更好的排列和形态以及增加的肌节长度,并在800 nm宽度凹槽上细胞结构的成熟度达到峰值。化学图案化过程将生物分子固定在基底表面,形成尺寸和位置可控的细胞易黏附区,这些生物分子一般是某些细胞外基质蛋白如纤粘连蛋白或者结合肽。这些图案可以通过微接触印刷和光刻技术制作。Salick等[65]发现宽度在30-80 μm的矩形条带微图案显著促进了人胚胎干细胞源心肌细胞的定向排列和肌节形成,且与条带长度无关。Werley等[66]设计了边长为50,100,250,500,1 000 μm的正方形微图案,人诱导多功能干细胞源心肌细胞在较大尺寸的微图案上显示出更高的电生理学和钙动力学功能成熟度。此外,他们进一步研究了不同几何形状的微图案对人诱导多功能干细胞源心肌细胞成熟度的影响,见图6。结果表明在区域5的细胞具有最大的成熟度。
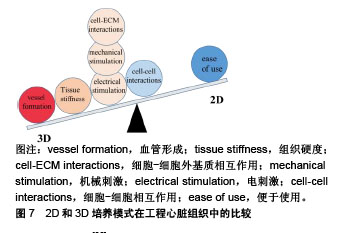
2.5 联合刺激 为了进一步促进体外干细胞源心肌细胞的成熟,可以联合机械刺激、电刺激、细胞-细胞外基质相互作用、细胞-细胞相互作用来模拟心肌在发育过程中的自然生理条件[78],见表1。
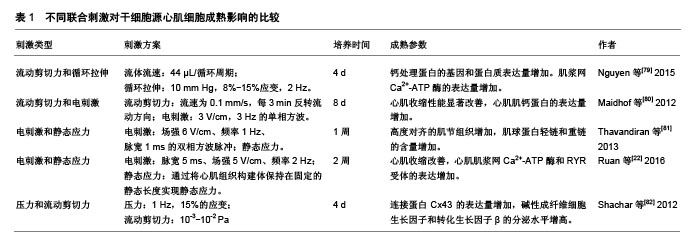

机械刺激:频率1 Hz、5%循环拉伸;电刺激:频率1 Hz、脉宽1 ms、强度3 V/cm的双相矩形脉冲。与静态对照相比,所有刺激组的构建体收缩力都较高;与单独的电刺激或机械刺激相比,联合机电刺激培养的工程心肌组织具有改善的功能特性;与同步机电刺激相比,延迟的联合机电刺激促进了心肌细胞的钙处理和肌钙蛋白的表达,因此电刺激和机械刺激组合的时机会影响其对工程化心脏组织的作用。
| [1] Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137(12):e67-e492.[2] Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America.Circulation. 2017;136(6):e137-e161.[3] Silva Dos Santos D, Brasil GV, Ramos IPR, et al. Embryonic stem cell-derived cardiomyocytes for the treatment of doxorubicin-induced cardiomyopathy.Stem Cell Res Ther. 2018;9(1):30.[4] Natsumeda M, Florea V, Rieger AC, et al. A Combination of Allogeneic Stem Cells Promotes Cardiac Regeneration.J Am Coll Cardiol. 2017; 70(20):2504-2515.[5] Ellison GM, Vicinanza C, Smith AJ, et al. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair.Cell. 2013;154(4):827-842.[6] Ben-Ari M, Naor S, Zeevi-Levin N, et al. Developmental changes in electrophysiological characteristics of human-induced pluripotent stem cell-derived cardiomyocytes.Heart Rhythm. 2016; 13(12):2379-2387.[7] Gorospe G, Zhu R, Millrod MA, et al. Automated grouping of action potentials of human embryonic stem cell-derived cardiomyocytes.IEEE Trans Biomed Eng. 2014;61(9):2389-2395.[8] Gherghiceanu M, Barad L, Novak A, et al. Cardiomyocytes derived from human embryonic and induced pluripotent stem cells: comparative ultrastructure.J Cell Mol Med. 2011;15(11):2539-2551.[9] Lieu DK, Liu J, Siu CW, et al. Absence of transverse tubules contributes to non-uniform Ca(2+) wavefronts in mouse and human embryonic stem cell-derived cardiomyocytes.Stem Cells Dev. 2009;18(10):1493-1500.[10] Kim C, Wong J, Wen J, et al. Studying arrhythmogenic right ventricular dysplasia with patient-specific iPSCs.Nature. 2013;494(7435):105-110.[11] Sheehy SP, Pasqualini F, Grosberg A, et al. Quality metrics for stem cell-derived cardiac myocytes.Stem Cell Reports. 2014;2(3):282-294.[12] Liu Z, Tang Y, Lü S, et al. The tumourigenicity of iPS cells and their differentiated derivates.J Cell Mol Med. 2013;17(6):782-791.[13] Gonzalez R, Lee JW, Schultz PG.Stepwise chemically induced cardiomyocyte specification of human embryonic stem cells.Angew Chem Int Ed Engl. 2011;50(47):11181-11185.[14] Zhao XL, Yang B, Ma LN, et al. MicroRNA-1 effectively induces differentiation of myocardial cells from mouse bone marrow mesenchymal stem cells.Artif Cells Nanomed Biotechnol. 2016;44(7):1665-1670.[15] Karakikes I, Senyei GD, Hansen J, et al. Small molecule-mediated directed differentiation of human embryonic stem cells toward ventricular cardiomyocytes.Stem Cells Transl Med. 2014;3(1):18-31.[16] Jacot JG, Martin JC, Hunt DL.Mechanobiology of cardiomyocyte development.J Biomech. 2010;43(1):93-98.[17] Bharadwaj KN, Spitz C, Shekhar A, et al. Computational fluid dynamics of developing avian outflow tract heart valves.Ann Biomed Eng. 2012; 40(10):2212-2227.[18] Tulloch NL, Muskheli V, Razumova MV, et al. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res. 2011;109(1):47-59.[19] Salameh A, Wustmann A, Karl S, et al. Cyclic mechanical stretch induces cardiomyocyte orientation and polarization of the gap junction protein connexin43.Circ Res. 2010;106(10):1592-1602.[20] Hove JR, Köster RW, Forouhar AS, et al. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis.Nature. 2003;421(6919):172-177.[21] Abilez OJ, Tzatzalos E, Yang H, et al. Passive Stretch Induces Structural and Functional Maturation of Engineered Heart Muscle as Predicted by Computational Modeling.Stem Cells. 2018;36(2):265-277.[22] Ruan JL, Tulloch NL, Razumova MV, et al. Mechanical Stress Conditioning and Electrical Stimulation Promote Contractility and Force Maturation of Induced Pluripotent Stem Cell-Derived Human Cardiac Tissue.Circulation. 2016;134(20):1557-1567.[23] Leonard A, Bertero A, Powers JD, et al. Afterload promotes maturation of human induced pluripotent stem cell derived cardiomyocytes in engineered heart tissues.J Mol Cell Cardiol. 2018;118:147-158.[24] Lux M, Andrée B, Horvath T, et al. In vitro maturation of large-scale cardiac patches based on a perfusable starter matrix by cyclic mechanical stimulation.Acta Biomater. 2016;30:177-187.[25] Zhang W, Kong CW, Tong MH, et al. Maturation of human embryonic stem cell-derived cardiomyocytes (hESC-CMs) in 3D collagen matrix: Effects of niche cell supplementation and mechanical stimulation.Acta Biomater. 2017;49:204-217. [26] Mihic A, Li J, Miyagi Y, et al. The effect of cyclic stretch on maturation and 3D tissue formation of human embryonic stem cell-derived cardiomyocytes.Biomaterials. 2014;35(9):2798-2808.[27] Qi Y, Li Z, Kong CW, et al. Uniaxial cyclic stretch stimulates TRPV4 to induce realignment of human embryonic stem cell-derived cardiomyocytes.J Mol Cell Cardiol. 2015;87:65-73.[28] Illi B, Scopece A, Nanni S, et al. Epigenetic histone modification and cardiovascular lineage programming in mouse embryonic stem cells exposed to laminar shear stress.Circ Res. 2005;96(5):501-508.[29] Geuss LR, Wu DC, Ramamoorthy D, et al. Paramagnetic beads and magnetically mediated strain enhance cardiomyogenesis in mouse embryoid bodies.PLoS One. 2014;9(12):e113982.[30] Thrivikraman G, Boda SK, Basu B.Unraveling the mechanistic effects of electric field stimulation towards directing stem cell fate and function: A tissue engineering perspective.Biomaterials. 2018;150:60-86.[31] Tandon N, Cannizzaro C, Chao PH, et al. Electrical stimulation systems for cardiac tissue engineering.Nat Protoc. 2009;4(2):155-173.[32] Radisic M, Park H, Shing H, et al. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds.Proc Natl Acad Sci U S A. 2004;101(52):18129-18134.[33] Liu J, Laksman Z, Backx PH.The electrophysiological development of cardiomyocytes.Adv Drug Deliv Rev. 2016;96:253-273.[34] Merrill DR, Bikson M, Jefferys JG.Electrical stimulation of excitable tissue: design of efficacious and safe protocols.J Neurosci Methods. 2005;141(2):171-198.[35] Baumgartner S, Halbach M, Krausgrill B, et al. Electrophysiological and morphological maturation of murine fetal cardiomyocytes during electrical stimulation in vitro.J Cardiovasc Pharmacol Ther. 2015;20(1):104-112.[36] Chan YC, Ting S, Lee YK, et al. Electrical stimulation promotes maturation of cardiomyocytes derived from human embryonic stem cells.J Cardiovasc Transl Res. 2013;6(6):989-999.[37] Chiu LL, Iyer RK, King JP, et al. Biphasic electrical field stimulation aids in tissue engineering of multicell-type cardiac organoids.Tissue Eng Part A. 2011;17(11-12):1465-1477.[38] Pietronave S, Zamperone A, Oltolina F, et al. Monophasic and biphasic electrical stimulation induces a precardiac differentiation in progenitor cells isolated from human heart.Stem Cells Dev. 2014;23(8):888-898.[39] Prado LN, Goulart JT, Zoccoler M, et al. Ventricular myocyte injury by high-intensity electric field: Effect of pulse duration.Gen Physiol Biophys. 2016;35(2):121-130.[40] Llucià-Valldeperas A, Sanchez B, Soler-Botija C, et al. Physiological conditioning by electric field stimulation promotes cardiomyogenic gene expression in human cardiomyocyte progenitor cells.Stem Cell Res Ther. 2014;5(4):93.[41] Hernández D, Millard R, Sivakumaran P, et al. Electrical Stimulation Promotes Cardiac Differentiation of Human Induced Pluripotent Stem Cells.Stem Cells Int. 2016;2016:1718041.[42] Sauer H, Rahimi G, Hescheler J, et al. Effects of electrical fields on cardiomyocyte differentiation of embryonic stem cells.J Cell Biochem. 1999;75(4):710-723.[43] Serena E, Figallo E, Tandon N, et al. Electrical stimulation of human embryonic stem cells: cardiac differentiation and the generation of reactive oxygen species.Exp Cell Res. 2009;315(20):3611-3619.[44] Ronaldson-Bouchard K, Ma SP, Yeager K, et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells.Nature. 2018;556(7700):239-243.[45] Chen MQ, Xie X, Wilson KD, et al. Current-Controlled Electrical Point-Source Stimulation of Embryonic Stem Cells.Cell Mol Bioeng. 2009;2(4):625-635.[46] Fujita H, Nedachi T, Kanzaki M.Accelerated de novo sarcomere assembly by electric pulse stimulation in C2C12 myotubes.Exp Cell Res. 2007;313(9):1853-1865.[47] Eng G Lee BW, Protas L, et al. Autonomous beating rate adaptation in human stem cell-derived cardiomyocytes.Nat Commun. 2016;7:10312.[48] Tandon N, Marsano A, Maidhof R, et al. Optimization of electrical stimulation parameters for cardiac tissue engineering.J Tissue Eng Regen Med. 2011;5(6):e115-125.[49] Hirt MN, Boeddinghaus J, Mitchell A, et al. Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation.J Mol Cell Cardiol. 2014;74:151-161.[50] Nunes SS, Miklas JW, Liu J, et al. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes.Nat Methods. 2013;10(8):781-787.[51] Sun X, Nunes SS.Biowire platform for maturation of human pluripotent stem cell-derived cardiomyocytes.Methods. 2016;101:21-26.[52] Zhu R, Blazeski A, Poon E, et al. Physical developmental cues for the maturation of human pluripotent stem cell-derived cardiomyocytes. Stem Cell Res Ther. 2014;5(5):117.[53] Besser RR, Ishahak M, Mayo V, et al. Engineered Microenvironments for Maturation of Stem Cell Derived Cardiac Myocytes.Theranostics. 2018;8(1):124-140.[54] Yang X, Pabon L, Murry CE.Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes.Circ Res. 2014; 114(3):511-523.[55] Engler AJ, Sen S, Sweeney HL, et al. Matrix elasticity directs stem cell lineage specification.Cell. 2006;126(4):677-689.[56] Jacot JG, Kita-Matsuo H, Wei KA, et al. Cardiac myocyte force development during differentiation and maturation.Ann N Y Acad Sci. 2010;1188:121-127.[57] Bajaj P, Tang X, Saif TA, et al. Stiffness of the substrate influences the phenotype of embryonic chicken cardiac myocytes.J Biomed Mater Res A. 2010;95(4):1261-1269.[58] Hazeltine LB, Simmons CS, Salick MR, et al. Effects of substrate mechanics on contractility of cardiomyocytes generated from human pluripotent stem cells.Int J Cell Biol. 2012;2012:508294.[59] McCain ML, Yuan H, Pasqualini FS, et al. Matrix elasticity regulates the optimal cardiac myocyte shape for contractility.Am J Physiol Heart Circ Physiol. 2014;306(11):H1525-1539.[60] Hazeltine LB, Badur MG, Lian X, et al. Temporal impact of substrate mechanics on differentiation of human embryonic stem cells to cardiomyocytes.Acta Biomater. 2014;10(2):604-612.[61] Wang PY, Thissen H, Kingshott P.Modulation of human multipotent and pluripotent stem cells using surface nanotopographies and surface- immobilised bioactive signals: A review.Acta Biomater. 2016;45:31-59.[62] Wanjare M, Huang NF.Regulation of the microenvironment for cardiac tissue engineering.Regen Med. 2017;12(2):187-201.[63] Wang PY, Yu J, Lin JH, et al. Modulation of alignment, elongation and contraction of cardiomyocytes through a combination of nanotopography and rigidity of substrates.Acta Biomater. 2011;7(9):3285-3293.[64] Carson D, Hnilova M, Yang X, et al. Nanotopography-Induced Structural Anisotropy and Sarcomere Development in Human Cardiomyocytes Derived from Induced Pluripotent Stem Cells.ACS Appl Mater Interfaces. 2016;8(34):21923-21932.[65] Salick MR, Napiwocki BN, Sha J, et al. Micropattern width dependent sarcomere development in human ESC-derived cardiomyocytes. Biomaterials. 2014;35(15):4454-4464.[66] Werley CA, Chien MP, Gaublomme J, et al. Geometry-dependent functional changes in iPSC-derived cardiomyocytes probed by functional imaging and RNA sequencing.PLoS One. 2017;12(3):e0172671.[67] Borovjagin AV, Ogle BM, Berry JL, et al. From Microscale Devices to 3D Printing: Advances in Fabrication of 3D Cardiovascular Tissues. Circ Res. 2017;120(1):150-165.[68] Lundy SD, Zhu WZ, Regnier M, et al. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells.Stem Cells Dev. 2013;22(14):1991-2002.[69] Liau B, Christoforou N, Leong KW, et al. Pluripotent stem cell-derived cardiac tissue patch with advanced structure and function.Biomaterials. 2011;32(35):9180-8187.[70] Zhang D, Shadrin IY, Lam J, et al. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials. 2013;34(23):5813-5820.[71] Huyer LD, Montgomery M, Zhao Y, et al. Biomaterial based cardiac tissue engineering and its applications.Biomed Mater. 2015;10(3):034004.[72] Alperin C, Zandstra PW, Woodhouse KA.Polyurethane films seeded with embryonic stem cell-derived cardiomyocytes for use in cardiac tissue engineering applications.Biomaterials. 2005;26(35):7377-7386.[73] LaNasa SM, Bryant SJ.Influence of ECM proteins and their analogs on cells cultured on 2-D hydrogels for cardiac muscle tissue engineering. Acta Biomater. 2009;5(8):2929-2938.[74] Reis LA, Chiu LL, Feric N, et al. Biomaterials in myocardial tissue engineering.J Tissue Eng Regen Med. 2016;10(1):11-28.[75] Ott HC, Matthiesen TS, Goh SK, et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart.Nat Med. 2008; 14(2):213-221.[76] Fong AH, Romero-López M, Heylman CM, et al. Three-Dimensional Adult Cardiac Extracellular Matrix Promotes Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes.Tissue Eng Part A. 2016;22(15-16):1016-1025.[77] Gao L, Kupfer ME, Jung JP, et al. Myocardial Tissue Engineering With Cells Derived From Human-Induced Pluripotent Stem Cells and a Native-Like, High-Resolution, 3-Dimensionally Printed Scaffold.Circ Res. 2017;120(8):1318-1325.[78] Scuderi GJ, Butcher J.Naturally Engineered Maturation of Cardiomyocytes. Front Cell Dev Biol. 2017;5:50.[79] Nguyen MD, Tinney JP, Ye F, et al. Effects of physiologic mechanical stimulation on embryonic chick cardiomyocytes using a microfluidic cardiac cell culture model.Anal Chem. 2015;87(4):2107-2113.[80] Maidhof R, Tandon N, Lee EJ, et al. Biomimetic perfusion and electrical stimulation applied in concert improved the assembly of engineered cardiac tissue.J Tissue Eng Regen Med. 2012;6(10):e12-23.[81] Thavandiran N, Dubois N, Mikryukov A, et al. Design and formulation of functional pluripotent stem cell-derived cardiac microtissues.Proc Natl Acad Sci U S A. 2013;110(49):E4698-4707.[82] Shachar M, Benishti N, Cohen S.Effects of mechanical stimulation induced by compression and medium perfusion on cardiac tissue engineering.Biotechnol Prog. 2012;28(6):1551-1559.[83] Morgan KY, Black LD 3rd.Mimicking isovolumic contraction with combined electromechanical stimulation improves the development of engineered cardiac constructs.Tissue Eng Part A. 2014;20(11-12):1654-1667.[84] Smith AS, Macadangdang J, Leung W, et al. Human iPSC-derived cardiomyocytes and tissue engineering strategies for disease modeling and drug screening.Biotechnol Adv. 2017;35(1):77-94.[85] Korolj A, Wang EY, Civitarese RA, et al. Biophysical stimulation for in vitro engineering of functional cardiac tissues.Clin Sci (Lond). 2017; 131(13):1393-1404.[86] Moretti A, Bellin M, Welling A, et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome.N Engl J Med. 2010;363(15): 1397-1409. |
| [1] | 蒲 锐, 陈子扬, 袁凌燕. 不同细胞来源外泌体保护心脏的特点与效应[J]. 中国组织工程研究, 2021, 25(在线): 1-. |
| [2] | 林清凡, 解一新, 陈婉清, 叶振忠, 陈幼芳. 人胎盘源间充质干细胞条件培养液可上调缺氧状态下BeWo细胞活力和紧密连接因子的表达[J]. 中国组织工程研究, 2021, 25(在线): 4970-4975. |
| [3] | 张秀梅, 翟运开, 赵 杰, 赵 萌. 类器官模型国内外数据库近10年文献研究热点分析[J]. 中国组织工程研究, 2021, 25(8): 1249-1255. |
| [4] | 万 然, 史 旭, 刘京松, 王岩松. 间充质干细胞分泌组治疗脊髓损伤的研究进展[J]. 中国组织工程研究, 2021, 25(7): 1088-1095. |
| [5] | 廖成成, 安家兴, 谭张雪, 王 倩, 刘建国. 口腔鳞状细胞癌干细胞的治疗靶点及应用前景[J]. 中国组织工程研究, 2021, 25(7): 1096-1103. |
| [6] | 谢文佳, 夏天娇, 周卿云, 刘羽佳, 顾小萍. 小胶质细胞介导神经元损伤在神经退行性疾病中的作用[J]. 中国组织工程研究, 2021, 25(7): 1109-1115. |
| [7] | 李珊珊, 郭笑霄, 尤 冉, 杨秀芬, 赵 露, 陈 曦, 王艳玲. 感光细胞替代治疗视网膜变性疾病[J]. 中国组织工程研究, 2021, 25(7): 1116-1121. |
| [8] | 焦 慧, 张一宁, 宋雨晴, 林 宇, 王秀丽. 乳腺癌类器官研究进展及临床应用前景[J]. 中国组织工程研究, 2021, 25(7): 1122-1128. |
| [9] | 王诗琦, 张金生. 中医药调控缺血缺氧微环境对骨髓间充质干细胞增殖、分化及衰老的影响[J]. 中国组织工程研究, 2021, 25(7): 1129-1134. |
| [10] | 曾燕华, 郝延磊. 许旺细胞体外培养及纯化的系统性综述[J]. 中国组织工程研究, 2021, 25(7): 1135-1141. |
| [11] | 孔德胜, 何晶晶, 冯宝峰, 郭瑞云, Asiamah Ernest Amponsah, 吕 飞, 张舒涵, 张晓琳, 马 隽, 崔慧先. 间充质干细胞修复大动物模型脊髓损伤疗效评价的Meta分析[J]. 中国组织工程研究, 2021, 25(7): 1142-1148. |
| [12] | 侯婧瑛, 于萌蕾, 郭天柱, 龙会宝, 吴 浩. 缺氧预处理激活HIF-1α/MALAT1/VEGFA通路促进骨髓间充质干细胞生存和血管再生[J]. 中国组织工程研究, 2021, 25(7): 985-990. |
| [13] | 史洋洋, 秦英飞, 吴福玲, 何 潇, 张雪静. 胎盘间充质干细胞预处理预防小鼠毛细支气管炎[J]. 中国组织工程研究, 2021, 25(7): 991-995. |
| [14] | 梁学奇, 郭黎姣, 陈贺捷, 武 杰, 孙雅琪, 邢稚坤, 邹海亮, 陈雪玲, 吴向未. 泡状棘球绦虫原头蚴抑制骨髓间充质干细胞向成纤维细胞的分化[J]. 中国组织工程研究, 2021, 25(7): 996-1001. |
| [15] | 樊全宝, 罗惠娜, 王丙云, 陈胜锋, 崔连旭, 江文康, 赵明明, 王静静, 罗冬章, 陈志胜, 白银山, 刘璨颖, 张 晖. 低氧培养犬脂肪间充质干细胞的生物学特性[J]. 中国组织工程研究, 2021, 25(7): 1002-1007. |
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
1.3 质量评估 共检索到英文文献300篇,通过题目、摘要筛除与文章研究目的无关及重复的文献,查阅全文收集到86篇英文文献,近5年文献占比约70%。文献筛选流程见图1。
.jpg)
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
.jpg)
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||