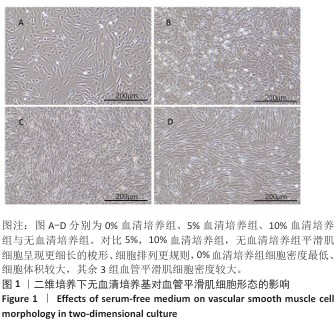
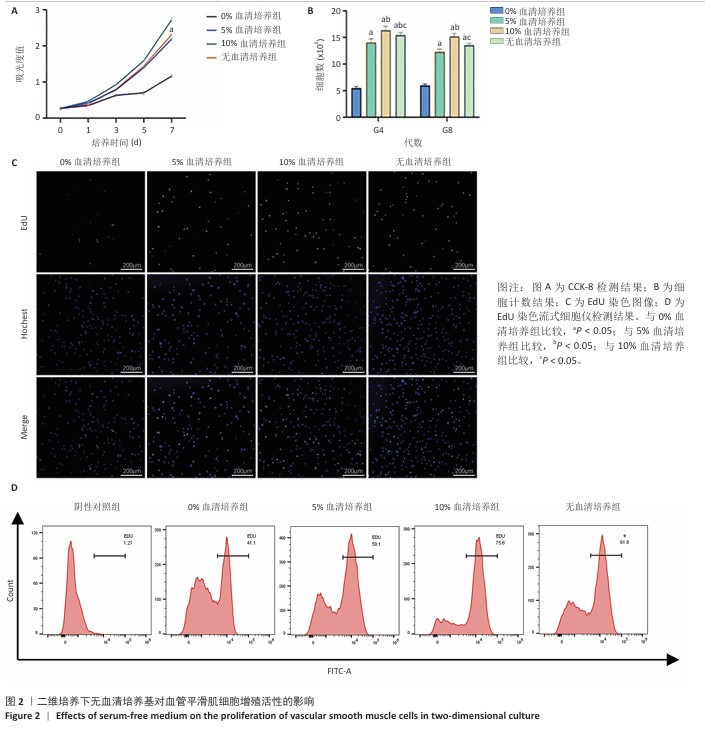
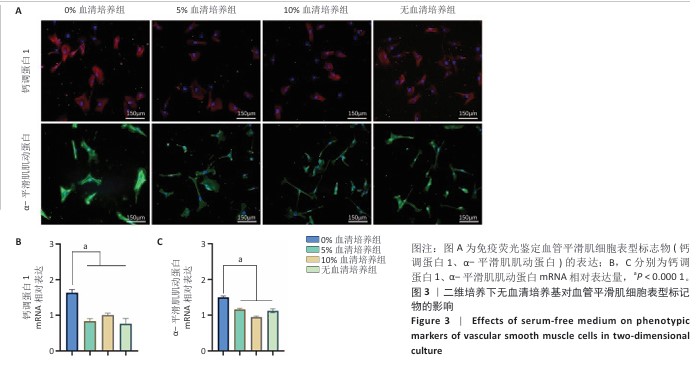
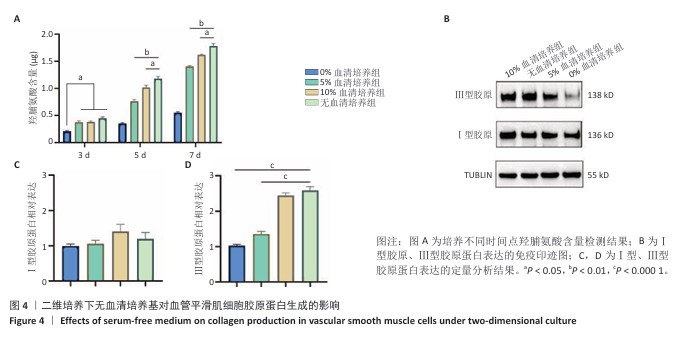
[1] KIRKTON RD, WATSON JDB, HOUSTON R 4th, et al. Evaluation of vascular repair by tissue-engineered human acellular vessels or expanded polytetrafluoroethylene grafts in a porcine model of limb ischemia and reperfusion. J Trauma Acute Care Surg. 2023;95(2):234-241.
[2] NIKLASON LE, LAWSON JH. Bioengineered human blood vessels. Science. 2020;370(6513):eaaw8682.
[3] SHAH MOHAMMADI M, BUCHEN JT, PASQUINA PF, et al. Critical considerations for regeneration of vascularized composite tissues. Tissue Eng Part B Rev. 2021;27(4):366-381.
[4] BILGEN B, ORSINI E, AARON RK, et al. FBS suppresses TGFbeta1-induced chondrogenesis in synoviocyte pellet cultures while dexamethasone and dynamic stimuli are beneficial. J Tissue Eng Regen Med. 2007;1(6):436-442.
[5] BRYAN N, ANDREWS KD, LOUGHRAN MJ, et al. Elucidating the contribution of the elemental composition of fetal calf serum to antigenic expression of primary human umbilical-vein endothelial cells in vitro. Biosci Rep. 2011;31(3):199-210.
[6] VAN DER VALK J, BIEBACK K, BUTA C, et al. Fetal bovine serum (FBS): past–present–future. ALTEX. 2018;35(1):99-118.
[7] BRUNNER D, FRANK J, APPL H, et al. The serum-free media interactive online database. ALTEX. 2010;27(1):53-62.
[8] GSTRAUNTHALER G, LINDL T, VAN DER VALK J. A plea to reduce or replace fetal bovine serum in cell culture media. Cytotechnology. 2013;65(5):791-793.
[9] KARNIELI O, FRIEDNER OM, ALLICKSON JG, et al. A consensus introduction to serum replacements and serum-free media for cellular therapies. Cytotherapy. 2017;19(2):155-169.
[10] DING S, WANG F, LIU Y, et al. Characterization and isolation of highly purified porcine satellite cells. Cell Death Discov. 2017;3(1):1-11.
[11] CANEPARO C, CHABAUD S, FRADETTE J, et al. Evaluation of a serum-free medium for human epithelial and stromal cell culture. Int J Mol Sci. 2022;23(17):10035.
[12] WU X, MA Z, WU D. Derivation of clinical-grade mesenchymal stromal cells from umbilical cord under chemically defined culture condition–platform for future clinical application. Cytotherapy. 2020;22(7):377-387.
[13] SKRIVERGAARD S, YOUNG JF, SAHEBEKHTIARI N, et al. A simple and robust serum-free media for the proliferation of muscle cells. Food Res Int. 2023;172:113194.
[14] KOLKMANN AM, VAN ESSEN A, POST MJ, et al. Development of a chemically defined medium for in vitro expansion of primary bovine satellite cells. Front Bioeng Biotechnol. 2022;10:895289.
[15] ROSS R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971;50(1):172-186.
[16] LIU X, ZHANG T, WANG R, et al. Insulin-transferrin-selenium as a novel serum-free media supplement for the culture of human amnion mesenchymal stem cells. Ann Clin Lab Sci. 2019;49(1):63-71.
[17] LIU X, LIU J, KANG N, et al. Role of insulin-transferrin-selenium in auricular chondrocyte proliferation and engineered cartilage formation in vitro. Int J Mol Sci. 2014;15(1):1525-1537.
[18] LUCHETTI CG, LORENZO MS, ELIA EM, et al. Effects of the addition of insulin–transferrin–selenium (ITS) and/or metformin to the in vitro maturation of porcine oocytes on cytoplasmic maturation and embryo development. Reprod Fertil Dev. 2023;35(5):363-374.
[19] SUN X, WANG N, JIANG H, et al. Insulin-transferrin-selenium promote formation of tissue-engineered vascular grafts in early stage of culture. Prep Biochem Biotech. 2024;54(9):1186-1195.
[20] FRANCIS GL. Albumin and mammalian cell culture: implications for biotechnology applications. Cytotechnology. 2010;62(1):1-16.
[21] SAKWE AM, KOUMANGOYE R, GOODWIN SJ, et al. Fetuin-A (α2HS-glycoprotein) is a major serum adhesive protein that mediates growth signaling in breast tumor cells. J Biol Chem. 2010;285(53): 41827-41835.
[22] ICER MA, YILDIRAN H. Effects of fetuin-A with diverse functions and multiple mechanisms on human health. Clin Biochem. 2021;88:1-10.
[23] LI Q. Pitfalls of protein extraction by RIPA buffer. Biotechniques. 2016;61(6):327-327.
[24] BOO YC. Ascorbic acid (vitamin C) as a cosmeceutical to increase dermal collagen for skin antiaging purposes: emerging combination therapies. Antioxidants. 2022;11(9):1663.
[25] VILLAGRAN M, FERREIRA J, MARTORELL M, et al. The role of vitamin C in cancer prevention and therapy: a literature review. Antioxidants. 2021;10(12):1894.
[26] ZHENG YY, ZHU HZ, WU ZY, et al. Evaluation of the effect of smooth muscle cells on the quality of cultured meat in a model for cultured meat. Food Res Int. 2021;150:110786.
[27] LIVINGSTON MJ, SHU S, FAN Y, et al. Tubular cells produce FGF2 via autophagy after acute kidney injury leading to fibroblast activation and renal fibrosis. Autophagy. 2023;19(1):256-277.
[28] BEKETOV EE, ISAEVA EV, YAKOVLEVA ND, et al. Bioprinting of cartilage with bioink based on high-concentration collagen and chondrocytes. Int J Mol Sci. 2021;22(21):11351.
[29] DAMANIK FF, ROTHUIZEN TC, VAN BLITTERSWIJK C, et al. Towards an in vitro model mimicking the foreign body response: tailoring the surface properties of biomaterials to modulate extracellular matrix. Sci Rep. 2014;4(1):6325.
[30] LUKJANENKO L, JUNG MJ, HEGDE N, et al. Loss of fibronectin from the aged stem cell niche affects the regenerative capacity of skeletal muscle in mice. Nat Med. 2016;22(8):897-905.
[31] SMITH J, RAI V. Novel factors regulating proliferation, migration, and differentiation of fibroblasts, keratinocytes, and vascular smooth muscle cells during wound healing. Biomedicines. 2024;12(9):1939.
[32] CHAKRABORTY R, CHATTERJEE P, DAVE JM, et al. Targeting smooth muscle cell phenotypic switching in vascular disease. JJVS Vasc Sci. 2021;2:79-94.
[33] MILLETTE E, RAUCH BH, DEFAWE O, et al. Platelet-derived growth Factor-BB–Induced Human smooth muscle cell proliferation depends on Basic FGF release and FGFR-1 activation. Circ Res. 2005;96(2):172-179.
[34] ALEXANDER MR, OWENS GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol. 2012;74(1):13-40. |